By: Andrea Rinaldi
Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
The first clinical safety trial on a tuberculosis drug since 2009 is now under way.
The phase I trial of TBA-354 will involve 50 volunteers from the United States, according to the TB Alliance, the not-for-profit product development partnership sponsoring the trial.
In preclinical studies, the compound showed more potent antibacterial and sterilising activity than pretomanid (PA-824), a related substance now in phase II and phase III clinical trials to assess its safety and efficacy, the alliance announced last week.
It said that the six years that passed between TBA-354 and the last drug to undergo Phase 1 trials shows that the pipeline of drugs to combat tuberculosis is disconcertingly empty.

Several promising TB medicines have reached phase II and phase III trials, but a more robust pipeline is needed to protect against drug resistance and reduce the time patients need for treatment, which at present ranges from six months to two years, the alliance said.
“While there has been considerable progress in pursuit of new TB drugs over the last decade, the global pipeline is still relatively small in comparison to what is needed to truly revolutionise TB treatment,” says Stephen Murray, the alliance’s senior medical officer. “While there are multiple products in late stage clinical trials, the risk of those products not making it to registration is a reality of drug development, and we must protect against that risk.”
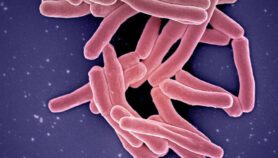
Tuberculosis remains one of the world’s deadliest communicable diseases. In 2013, an estimated nine million people contracted TB and there were 1.5 million deaths from the disease, according to WHO.
“One of the most exciting facets of the TB drug development field is the amount of work that is conducted by local researchers in endemic regions, especially in the clinical research stages.”
Stephen Murray, TB Alliance
Due to the scale of this problem, some researchers in the field say the trial of TBA-354 is likely to have only a modest impact despite being an important step. “We must keep in mind that the process of drug development takes a long time,” warns Giovanna Riccardi, a microbiologist from the University of Pavia in Italy. “In order to have a continuous delivery of TB medicines, we need to fill the gap that currently exists between the last stage of preclinical development and advanced clinical trials.”
To deliver the medicines required and reduce TB deaths, private sector research must be intensified and both public and private partners should spend more on international research collaborations, the TB Alliance said.
This means including more researchers in developing countries in privately funded later-stage clinical trials, says Murray, as these trials are often too expensive for developing countries to host on their own.
“One of the most exciting facets of the TB drug development field is the amount of work that is conducted by local researchers in endemic regions, especially in the clinical research stages,” he says. “South Africa, for example, is the epicentre of clinical TB research, and has local researchers and centres that may be the most advanced in the world.”