Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
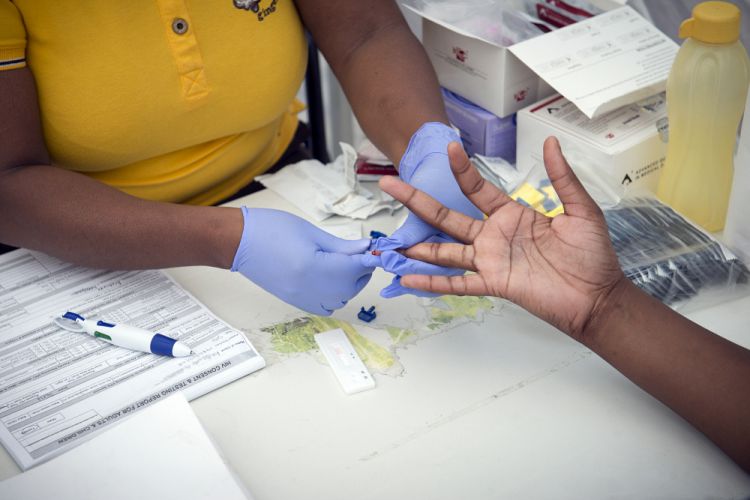
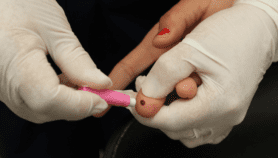
[DURBAN] People at high risk of contracting a HIV infection could be protected by taking anti-retroviral medicines (ARV) regularly, a conference in Durban, South Africa, has heard.
The studies were presented at the 21st International AIDS Conference in South Africa last month (18-22 July).
The so-called ASPIRE trial, undertaken by the United States’ Microbicide Trials Network, delivered a monthly vaginal ring coated in dapivirine, an ARV, to 2,600 HIV-negative women from Malawi, South Africa, Uganda and Zimbabwe. Earlier this year, results showed the monthly ring reduced the risk of infection by 25 per cent. After further analyses, that figure has now risen to more than 50 per cent.
Jared Baeten, a professor of global health at the US-based University of Washington in Seattle, and ASPIRE chair, says the ring could offer significant protection.
“It’s reduced the risk by half in women over 21. There’s also a correlation between protection and dosage. The higher the dosage and use, the higher the protection.” Baeten explains.
Plus Pills, a study to provide pre-exposal prophylaxis involving oral use of Truvada, a combination drug with the ARVs tenofovir and emtricitabine, among South African adolescents at high risk of HIV, found no new HIV case, said Katherine Gill, a medical officer at the South Africa-based Desmond Tutu HIV Centre. According to Gill, pre-exposal prophylaxis could be a viable alternative to condoms.
Zeda Rosenberg, chief executive officer of the International Partnership for Microbicides, says they intend to submit the vaginal ring for regulatory approval in the United States by 2017, and that the product could be available for public use by 2018.
Rosenberg calls for an urgent need to expand pre-exposure prophylaxis for women: “More than half of the women in the communities the trials took place will likely be infected within ten years without prophylaxis,” she says.
But she advises that HIV infections could only be reduced by offering multiple options that fit into women’s lives.
This piece was produced by SciDev.Net’s Sub-Saharan Africa English desk.