Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
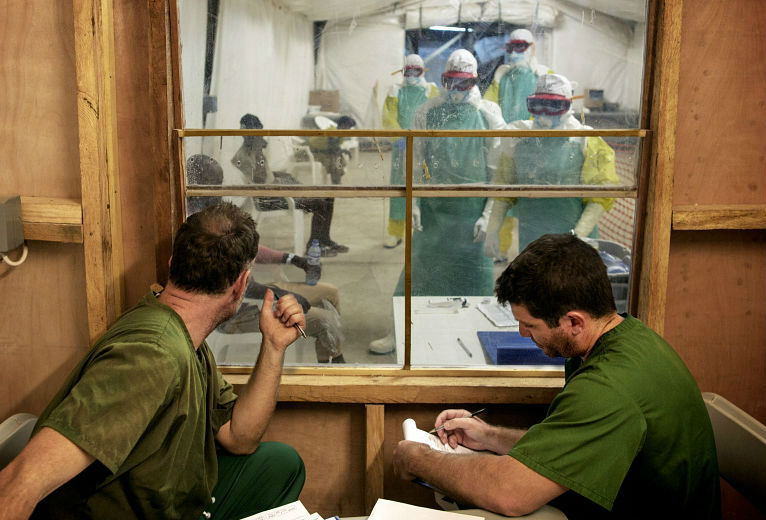
Those fighting Ebola may soon have a major weapon against the virus. An Ebola vaccine has been shown to be “highly efficacious” in protecting against the disease, according to the results of a fast-track clinical trial in Guinea, published in medical journal The Lancet today.
“This new vaccine, if the results hold up, may be the silver bullet against Ebola, helping to bring the current outbreak to zero and to control future outbreaks of this kind,” says Børge Brende, the minister of foreign affairs of Norway, which provided some of the trial’s funding.
The vaccine, called rVSV-ZEBOV, is the first one to reach this stage of clinical testing and to show such promising results — in less than a year. “Normally, it takes about a decade or more for a vaccine to come to this point,” says Peter Smith, a tropical epidemiologist at the London School of Hygiene & Tropical Medicine in the United Kingdom, who was involved in the trial’s design.
The vaccine is made up of virus components that are too weak to cause disease, but do stimulate the body’s immune response to protect patients against infection. It was tried on about 3,500 people in close contact with the Ebola virus in Guinea, West Africa, between 1 April and 20 July.
“This may be the silver bullet against Ebola, helping to bring the current outbreak to zero and to control future outbreaks of this kind”
Børge Brende, the minister of foreign affairs of Norway
Patients were split randomly into two groups: 2,000 patients were vaccinated immediately, while another 1,500 received the vaccine three weeks later. After ten days, there were no Ebola cases in the immediate vaccination group, compared with 16 cases in the delayed vaccination group, “showing a vaccine efficacy of 100 per cent”, the paper says. The study did not look at the effect of the vaccine on people who were already infected.
The researchers say the results could be applicable to other regions of Guinea, as well as to Liberia and Sierra Leone, the other two countries most affected by the epidemic in West Africa.
rVSV-ZEBOV “will likely be used in at-risk communities where and when it is required” rather than in large-scale immunisation campaigns, says Mark Feinberg from the company MSD Vaccines, which made the vaccines. It could also help protect health workers who care for Ebola patients, Feinberg adds.
But the vaccine still has to overcome several hurdles before it can be administered widely. “This is not a very friendly vaccine for common use” as it has to be kept at -70 degrees Celsius, says Smith. “You can do that in the context of a trial, but it’s much harder in routine circumstances” in tropical countries with unreliable electricity supplies to power refrigerators.
rVSV-ZEBOV will also need formal approval by national bodies. Feinberg says more research is under way “to provide a comprehensive set of data to inform licensure decisions”. Smith says the results “looks very exciting, but the numbers [of people in the trial] are small, so regulatory agencies might require somewhat stronger evidence.”
The trial was modelled on the ring vaccination method. This means the vaccine was administered to people at high risk of infection, because they live in the same household as an Ebola patient or were in contact with a patient’s body. “Contacts of contacts”, such as neighbours or family members living nearby, were also included.While ring vaccination was used in the 1970s to eradicate smallpox with a vaccine that was already known to be effective, it is the first time it has been used to evaluate the efficacy of a candidate vaccine, says Smith.
In contrast, separate efforts to test other candidate vaccines “have used a more static approach” that involves waiting for cases to occur in a given population, says Smith. But as Ebola declined in the region, cases became rarer, making it harder to find enough patients to enrol in these clinical trials. The ring vaccination method allowed the trial team to work around this problem by following the epidemic as it dispersed into smaller, local outbreaks.
References
Henao-Restrepo and others Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial (The Lancet, 31 July)