By: Lamine Konkobo
Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
An international group of scientists is pushing a groundbreaking DNA editing programme known as “mosquito gene drive” for a long-term solution to the spread of malaria in Africa.
Malaria, which is a preventable and a treatable disease, remains a leading cause of death in the world’s hottest regions, including most of Sub-Saharan Africa. In 2015, there were roughly 212 million cases of the disease worldwide and an estimated 429,000 deaths, according to the World Health Organization (WHO).
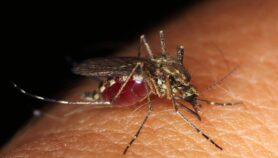
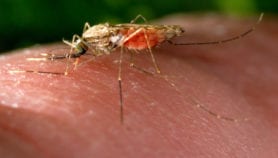
The Anopheles mosquito that transmits malaria feeds on infected people and picks up the Plasmodium parasite that causes the disease. The parasite then develops in the mosquito for about a week or two. When it feeds on another person, it passes on the malaria parasite.
As part of the mosquito gene drive project, scientists are working to genetically create a new population of mosquitoes that would be unable to spread the parasite that causes malaria, resulting in the eradication of the deadly disease.
However, as with any scientific research involving DNA editing, the programme raises concerns, with some scientists saying total openness and transparency are necessary.
A novel technology
The mosquito gene drive project is being conducted by the Target Malaria initiative, a not-for-profit research consortium that aims to develop and share novel technologies for malaria control.
Target Malaria is hosted at Imperial College London in the United Kingdom, with partner teams in Burkina Faso, Mali and Uganda.
“We are working in our laboratories in London to develop a mosquito whose reproductive capacities are modified,” Austin Burt, the global principal investigator of Target Malaria, told SciDev.Net. “By either reducing female mosquito fertility, or increasing the ratio of males to females, we hope to reduce the population of malaria-carrying mosquitoes to the point where malaria transmission is interrupted.”
Gene drive is a technique that allows scientists to speed up the evolutionary process of a species.
To modify the Anopheles mosquito, the scientists are investigating the use of genes which, at a cellular level, produce a biochemical substance (or enzyme) called nuclease. The production of nuclease sets off a process which disrupts specific sequences of DNA.
"When introduced in the malaria mosquito, they [the nucleases] work by identifying and cutting through essential genes targeted by our researchers, such as fertility genes," Burt says. "The interrupted gene will no longer function, and modified mosquitoes will be affected according to the nature and importance of the gene."
The ultimate goal of all the strategies involved in the research is to produce modified malaria mosquitoes that can pass on their new genetic makeup to a “disproportionately” high percentage of their offspring.
It is the artificial mechanism of achieving genetic modification that quickly cascades throughout a population — in this case, Anopheles mosquitoes — which is called gene drive.
In other words, gene drive is a technique that allows scientists to speed up the evolutionary process of a species.
With this speedy technique, instead of having the normal rate of inheritance (or Mendelian Inheritance), where a gene is passed on to the offspring 50 per cent of the time, the genetic inheritance occurs at a much higher rate.
"To this date, we have proven in our laboratories in London that we can pass a gene to up to 95 per cent of the progeny," Burt says.
Long-term solution
Fighting malaria by permanently interrupting the way the disease is spread by mosquitoes using genetics has never been attempted before. The researchers hope it will provide a sustainable and cost effective malaria prevention tool.
But as things stand, the GD programme is not expected to be saving people from malaria for at least another 10 years. This is because it involves a number of steps.
"We conduct our research around three main stages," Burt says. "In the first stage, we are producing a mosquito strain where males are sterile. The primary aim of this first stage is to allow us to validate some of our expectations and build capacity and operational experience amongst the teams."
The team has now made it past this first stage. Today, they are able to switch on a fertility-disabling enzyme in male Anopheles mosquitos, which prevents fertilisation of an egg after mating with a female.
“To this date, we have proven in our laboratories in London that we can pass a gene to up to 95 per cent of the progeny.”
Austin Burt, Target Malaria
However, the scientists have yet to figure out a way to pass on to future generations of mosquitoes this new genetic property that interrupts fertility. That is because the goal is not just to cause ‘infertility’ among male mosquitoes but to reduce — if not wipe out — the female mosquito population.
"In the second stage, we will progress to self-limiting but fertile males producing male-biased offspring — meaning that most (more than 90 per cent) of the progeny will be males,” Burt told SciDev.Net. “This is an interim stage to evaluate the safety and efficacy of our technology," he added.
Eventually, the researchers hope to progress to developing modified male mosquitoes that are fertile and able to pass on a genetic change that becomes permanent.
They are looking at two options to achieve this. The first one is to develop fertile male mosquitoes which produce male-dominated offspring; the second is to develop fertile males carrying a gene that will spread through the mosquito population, and as it does so, cause females that inherit the gene from both parents to be sterile.
African connection
The Target Malaria team is working to modify only the mosquito species that are most important for malaria transmission in Africa. To do that, it collaborates with three partner institutions in Africa: The Malaria Research and Training Center in Mali, the Uganda Virus Research Institute in Uganda and the Institut de Recherche en Sciences de la Santé in Burkina Faso.
Abdoulaye Diabate is heading the work in Burkina Faso out of the country's second city, Bobo-Dioulasso Diabate and his colleagues are conducting extensive field studies, experimenting on a sterile male mosquito strain to better understand its behaviour.
“A single group can't do all the work,” Diabate told SciDev.Net. “[Developing] the technology requires different experts with different backgrounds. All these different backgrounds are within my team here and within the different teams in Africa.”
“Any technology that will be introduced in Africa to combat malaria must work with the genetic background of mosquitoes here in the field.”
Abdoulaye Diabate, Institut de Recherche en Sciences de la Santé
The African partners within the Target Malaria consortium operate in a region where malaria is a major health issue — so they have access to the disease-transmitting mosquito, and can study it closely. They can also provide entomological data to guide the development of the gene drive technology.
“Any technology that will be introduced in Africa to combat malaria must work with the genetic background of mosquitoes here in the field," Diabate says.
But the modification journey starts in the United States.
"Basically, what is happening is that, all the way from Seattle where they are engineering the protein [for the genetic modification], they send it to Imperial College London where they do the genetic transformation,” explains Diabate. “Then, they will test these [new] mosquitoes along a really long pathway, making sure all the different [blueprints] the mosquitoes are responding to are quite clear."
Once this preliminary experiment is done in London, the resulting GM mosquitoes are sent to the teams in the field in Africa where tests will be run to ensure that the transgenic lines work with the genetic backgrounds of the local mosquitoes.
Diabate’s team aims to anticipate the possible behaviour of the GM mosquitoes once they have been released in the wild — which is a long way off.
"If you want to take these mosquitoes to the field one day, basically you need to understand what are the exact characteristics of the field type of mosquitoes, in terms of not just malaria transmission, but also the seasonal variations of the species that are transmitting malaria," he says. "It will take about ten years before we are ready to take the gene drive construct to the field."
Diabate says African scientists have substantial involvement in the development of the technology, dismissing any notion that they have no control over it owing to the fact that the Consortium has a foreign funder, the Bill and Melinda Gates Foundation.
"From the inception of the gene drive technology to the deployment in the field and in every single aspect, we are involved and we have our say,” he told SciDev.Net. "In no way [has] this technology been imposed on us. We are working with all the experts within the consortium."
Diabate adds that African countries have to look at the balance of risks and benefits involved in using the technology.
But how can everyone involved determine the level of risk?
Serious concerns
The eventual use of gene drive to prevent mosquitoes from spreading malaria is one tiny possibility out of many — if not unlimited — possibilities of this technology.
Apart from the possible intentional misuse that could be made of it, the possibility of catastrophic errors leading to unpredictable scenarios is causing concerns.
“When it comes to transgenic material, there is still in many some scepticism,” Diabate says, acknowledging the concerns. “This is a new technology and not all scientists are familiar with it, even in Europe and in the US.”
“Any experiments seeking to build gene drive systems that would spread in a wild species must not be performed in the ecosystem harbouring that species. […] From a safety perspective, it's simply unwise.”
Kevin Esvelt, MIT
The general concern about gene editing is certainly more than a simple matter for fretful sceptics. The handling of this untested technology requires safeguarding, according to Kevin Esvelt, principal investigator of gene drive research at the Massachusetts Institute of Technology (MIT) in the United States.
“Any experiments seeking to build gene drive systems that would spread in a wild species must not be performed in the ecosystem harbouring that species. […] From a safety perspective, it's simply unwise,” Esvelt told SciDev.Net.
It is because of this specific safety concern that Target Malaria's research is done in London, far from the ecosystem of the Anopheles gambiae mosquitoes which is the African species responsible for transmitting malaria — and the species being used for the gene drive push.
In other words, Target Malaria's research would have a great potential ecological risk had it ran its gene editing experiment directly on the continent.
“Any escape from an African laboratory could result in the drive system spreading to eventually edit most or all African mosquitoes of the target species. Regardless of whether it worked against malaria, such an outcome would be a travesty on many levels,” Esvelt says.
Scientists say that as things stand, no conditions are safe enough for a field trial of a non-local gene drive system, a fact which Burt and his team are well aware of. “Our technology will undergo a fully independent risk assessment as part of the research process, and specific studies are carried out in laboratories to test for possible impacts,” he says.
“The research is proceeding step by step and we are working with regulatory authorities, external advisors and local communities to identify and address concerns and potential risks,” he adds. “Safety is paramount to our teams [and] we will only go forward if the technology is acceptable.”
Kevin Esvelt, who vowed to take a vocal public stand against any reckless gene drive research, is confident with the precautions taken by scientists at Target Malaria.
“The best way to find out whether using a gene drive to suppress populations of mosquitoes is good or bad is to suppress the local mosquito population WITHOUT using a gene drive and see what happens,” Esvelt says. “That's what Target Malaria is currently working towards.”
“This has basically zero ecological risk because the males are sterile and any ecological effect would be temporary since stopping releases would halt suppression.”
Kevin Esvelt, MIT
He explains that the suppression starts with the team’s constructing a strain of Anopheles gambiae mosquitoes for which the males are sterile — and since mosquitoes mate only once, releasing sterile males suppresses the local population.
“This has basically zero ecological risk because the males are sterile and any ecological effect would be temporary since stopping releases would halt suppression,” Esvelt says.
With necessary safeguarding, it's far better to edit a mosquito species so that it can no longer effectively spread malaria than to soak a continent in pesticides such as DDT, he adds.
Full transparency
Esvelt urges transparency in gene drive programmes, and believes his Target Malaria colleagues may now be more open about their work than they were earlier in the process.
“I've clashed with the Target Malaria team on the issue of openness before — though more with their communications people and strategists rather than their scientists,” he says. “But our differences centred on their keep-the-project-quiet-and-avoid-attention approach pre-2015, with respect to the West and the mainstream media, not Africans.”
“We are completely confident that if this technology is going to fail, it is not going to be for the science per se, but I think it is mostly going to be about the communication — so we need to make sure we do as much work building trust with people.”
Abdoulaye Diabate, Institut de Recherche en Sciences de la Santé
The researchers respond by saying that openness and building trust are part of the process of developing the technology.
"We are really open to people. In no way are we trying to hide anything,” Diabate, principal Investigator of the team in Burkina Faso, says. “We are completely confident that if this technology is going to fail, it is not going to be for the science per se, but I think it is mostly going to be about the communication — so we need to make sure we do as much work building trust with people.”
Citing the example of his interview with SciDev.Net, Diabate says his team replies to solicitations whenever their load of work does not come in the way.
“If you are really completely open to people, showing them you don't have anything to hide, that you want to work with them in full transparency, then you give them a voice to understand their concerns which you can factor into the building process of the technology, and that really helps a lot,” Diabate says.
While the researchers are addressing issues of openness and building trust with communities, they are still struggling with some concerns, such as whether there is going to be resistance to the gene drive once it is deployed in the field years down the road.
“Africans must weigh the consequences of doing nothing — a thousand children dying every single day — against the possible ecological side-effects.”
Kevin Esvelt, MIT
Between these unanswered questions and the uncertainty about the ecological risks, the team is facing a long journey. But for a durable solution against malaria, scientists agree that pushing the gene drive programme is worth all its difficulties.
“Africans must weigh the consequences of doing nothing — a thousand children dying every single day — against the possible ecological side-effects,” Kevin Esvelt says.
“DDT was an environmental disaster, but one of the greatest lifesaving interventions ever. Was it worth it? To the people who would otherwise have died from malaria, certainly. To everyone else? Debatable. Because it narrowly targets a single species, gene drive will have many fewer side-effects than DDT.”
This article was originally published by SciDev.Net’s Sub-Saharan Africa French edition.