By: Priya Shetty
Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
Priya Shetty explores the tools and partnerships that help the public health community counter the threat of counterfeit medicines.
Counterfeiting is as old as industrialisation. For as long as the idea of intellectual property or branding has existed, counterfeiters have schemed to cheaply mimic products for profit.
Far from being merely a nuisance, counterfeit medicines can cause serious illness and even death.
The trade in counterfeit drugs has grown into a global industry worth billions of dollars, targeting mostly developing countries [see Table 1]. The World Health Organization (WHO) estimates that 10–30 per cent of the medicines on sale could be fake in the developing world; the proportion is probably higher in some parts of Africa, Asia and Latin America. [2]
But public health experts believe poor regulatory and surveillance systems in the developing world mean the problem is even more widespread than it seems. This has spurred the global health community into action, with agencies including the WHO and Interpol launching initiatives to fight drug counterfeiting.
|
Trade name |
Report |
Country, year of detection |
|
Anti-diabetic traditional medicine (used to lower blood sugar) |
Contained six times the normal dose of glibenclamide (two people died, nine people hospitalized) |
China, 2009 |
|
Metakelfin (antimalarial) |
Fake versions discovered in 40 pharmacies lacked sufficient active ingredient |
United Republic of Tanzania, 2009 |
|
Viagra and Cialis (for erectile dysfunction) |
Fake versions smuggled into Thailand from an unknown source in an unknown country |
Thailand, 2008 |
|
Xenical (for fighting obesity) |
Fake versions sold into the US via websites operated elsewhere, but contained no active ingredient |
United States of America, 2007 |
Table 1: Counterfeit drug detections
Adapted from [1]
Blurred boundaries, clear risks
A key challenge in tackling counterfeiting lies in subtle differences between fake and substandard drugs. [3]
Many fake medicines are dummies deliberately created to resemble genuine drugs. Often they are utterly devoid of any active ingredient — but sometimes they may contain harmful or poisonous chemicals.
Substandard drugs do have some medicinal value, but may contain far lower doses of the active ingredient than they should. They are usually the product of negligent manufacturing and poor quality control rather than malicious criminal activity.
But both fake and substandard drugs have an immediate impact on patients who don’t receive the treatment they need. And more so in the case of substandard medicines, they can also have the disastrous side-effect of increasing resistance to treatment for serious diseases.
For instance, if a patient with malaria takes antimalarial drugs that contain a weak dose of the active ingredient, the malaria parasite will only be partially cleared from the body. The parasites that remain will be those that have resisted the drug. When these multiply and go on to infect new individuals, drug resistance spreads.
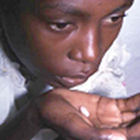
Pills that contain a weak dose of antimalarial fuel drug-resistant disease
WHO_TDR/Crump
To complicate matters further, legal definitions of counterfeit medicines are often so broad that they include generic drugs. [4] Generics are non-brand versions of pharmaceutical drugs that are produced cheaply either after the brand exclusivity has expired, or through special licensing laws. Millions of people rely on inexpensive generic drugs to fight potentially fatal diseases such as malaria or HIV/AIDS. Counterfeiting laws that blur the boundary with these essential drugs can seriously threaten access to life-saving medicines.
Why is counterfeiting flourishing?
There are several reasons why counterfeit drugs are most common in developing countries.
Many rural areas have few pharmacies or health clinics, and the ones that exist are often open only irregularly. Many people buy drugs in non-regulated outlets, such as markets, which are more likely to trade in counterfeits.
Fake drugs are often sold more cheaply, appealing to poor people for whom cost is a huge barrier to the healthcare they need. And often, both stolen and ‘knock-off’ goods are widely and openly sold in markets in countries such as Thailand — so counterfeit drugs may be mistaken for stolen, and therefore cheap, genuine medicines.
The dismally poor legislative and regulatory framework monitoring drug quality and sale in developing countries also allows counterfeiting to thrive. Even when counterfeiters are caught, the penalties tend to be far lower than for smuggling heroin or cocaine, for instance. [5]



Fake drugs are often sold cheaply in markets, making them an attractive option for poor people
Flickr/Zalazdore
Meanwhile, globalisation eases the way for counterfeiting, spreading distribution networks and making them more complex. As a result, drugs are harder to track. And it is no surprise that the Internet has made selling counterfeit drugs far easier, both in developed and developing nations. The European Alliance for Access to Safe Medicines suggests that over 60 per cent of prescription medicines sold through the Internet are fakes. [6]
Advances in technology have also made high-quality labels and packaging relatively easy to produce, and pharmaceutical chemicals cheap to mass-produce.
How to spot a counterfeit
There are sometimes very obvious telltale signs of counterfeiting — faulty spelling, for example, incorrect packaging or tablet size.
Yet counterfeiters are fast becoming better at replicating genuine drugs correctly, and are increasingly sophisticated when mimicking specific anti-counterfeit measures such as brand logos. This has pushed manufacturers to enhance anti-counterfeit technology (panel 1). [7]
Panel 1: Anti-counterfeit technologies
Visible markings
Medicines can be marked in ways that make it easy even for consumers to identify fakes. For instance, packets and bottles can have tamper-evident seals. And like banknotes and credit cards, medicines can be marked with embossed graphics, or holograms — security inks that change in colour according to the angle they are viewed at.
Invisible markings
These are usually identifiable only to the supplier or distributor, not the consumer. They make use of invisible inks that can be detected in UV light, digital watermarks that encode data in graphics, anti-scan designs that reveal a watermark when copied, or inks imbued with specific micro-encapsulated odours.
Forensic labelling
Manufactures can use ‘lock and key’ systems, applying specific biological or chemical tags, such as DNA, that are not detectable by standard analysis and can only be revealed by specific reagents. Other forms of tagging can include silicon dioxide micro or nano tags, applied to the surface of a pill, that emit a unique light signature.
Track and trace labelling
In this system, each pharmaceutical package is uniquely labelled either with a barcode or some other non-sequential unique number. The label should then be read at the final point in the supply chain, when a pharmacist dispenses medicines to a consumer. This helps to ensure drugs are genuine and not past their expiration date. A more complex system is radio frequency identity (RFID) tagging, in which the tag is an antenna with a microchip. This means that the data can be read at a greater distance and do not need to be scanned like a barcode.
Adapted from [7]
Who is fighting the counterfeit trade?
In 2006, the WHO set up the International Medical Products Anti-Counterfeiting Taskforce (IMPACT). This Taskforce has been leading international action, offering guidance on how to strengthen legislative and regulatory frameworks. Through IMPACT, the WHO has joined forces with regulatory agencies such as Interpol (panel 2) to uncover counterfeit operations.
Panel 2: Interpol: Chasing counterfeits across the world
In 2010, Operation Pangea III, coordinated by Interpol and IMPACT, collated data from 45 participating countries and uncovered a vast network of counterfeit drug sales on the Internet. The operation revealed 694 websites engaged in illegal activity, 290 of which have now been shut down.
Customs seized over 1 million counterfeit pills worth a total of US$2.6 million. These pills included antibiotics, steroids, anti-cancer, anti-depression and anti-epileptic pills, as well as slimming or food supplement tablets.
This success followed on from Operation Storm II, carried out earlier in 2010, which targeted eight countries across South-East Asia: Cambodia, China, Indonesia, Laos, Myanmar, Singapore, Thailand and Vietnam. That led to the seizure of 20 million fake medicines including antibiotics, anti-malarial and birth control tablets, anti-tetanus serums, aspirin and erectile dysfunction drugs. Over 100 pharmacies and illicit drug outlets were shut down.
The Medicines Transparency Alliance (MeTA) — launched in 2008 with support from the UK Department for International Development (DFID), the WHO and the World Bank — has set up multi-stakeholder forums to examine every aspect of the medicines supply chain in seven pilot countries: Ghana, Jordan, Kyrgyzstan, Peru, the Philippines, Uganda and Zambia.
Counterfeiting seriously infringes on intellectual property, and since patents for medicines tend to be held by drug manufacturers, they are heavily involved in fighting the trade in fake drugs.
In some cases, the only way to detect a counterfeit drug is through chemical analysis. Since the pharmaceutical industry has a vested interest in quickly detecting fake drugs, several companies have sent mini-labs around countries in Africa and Asia (especially China) to assess the quality and ingredients in drugs.
The pharmaceuticals industry has also formed an alliance called the Pharmaceutical Security Institute (PSI), which counts 21 R&D-focused drug manufacturers as members. PSI works with the WHO and Interpol to exchange information on counterfeiting operations.
The problem is that strict anti-counterfeiting operations and vague legislation tend to target essential generics too.
Some scientists are very concerned about a new treaty to combat counterfeit drugs, the Council of Europe’s Medicrime Convention. Signed in December 2010, this treaty criminalises the manufacture and trade in counterfeit medicines and contains a definition of counterfeiting so broad that most generics could come under its banner. [8]
To fight counterfeit medicines effectively, governments will need to focus on a few key areas.
The foremost of these is inter-country collaboration, since much of the trade in fake drugs occurs across national borders. Pharmacies, hospitals and other points in the supply chain need to keep lines of communication open and to exchange information.
And the entire medical supply chain will need to be tightened to eliminate loopholes in surveillance and monitoring. Legislation will also need to be updated and clarified to keep pace with the scale of the problem, and to provide a sufficient deterrent to criminal activity.
Finally, the global health community and national governments will need to engage with consumers so that patients understand the importance of buying medicines through regulated outlets.
But for any of these initiatives against counterfeit drugs to work, countries need a strong dose of political will — enough to strengthen the legal regulatory framework, improve education and invest in technologies that can detect counterfeit drugs and prevent further damage to public health.



Journalist Priya Shetty specialises in developing world issues including health, climate change and human rights. She writes a blog, Science Safari, on these issues. She has worked as an editor at New Scientist, The Lancet and SciDev.Net.
This article is part of a Spotlight on Detecting counterfeit drugs.