23/02/23
Preventative antibiotic during labour ‘saves lives’
By: Claudia Caruana
Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
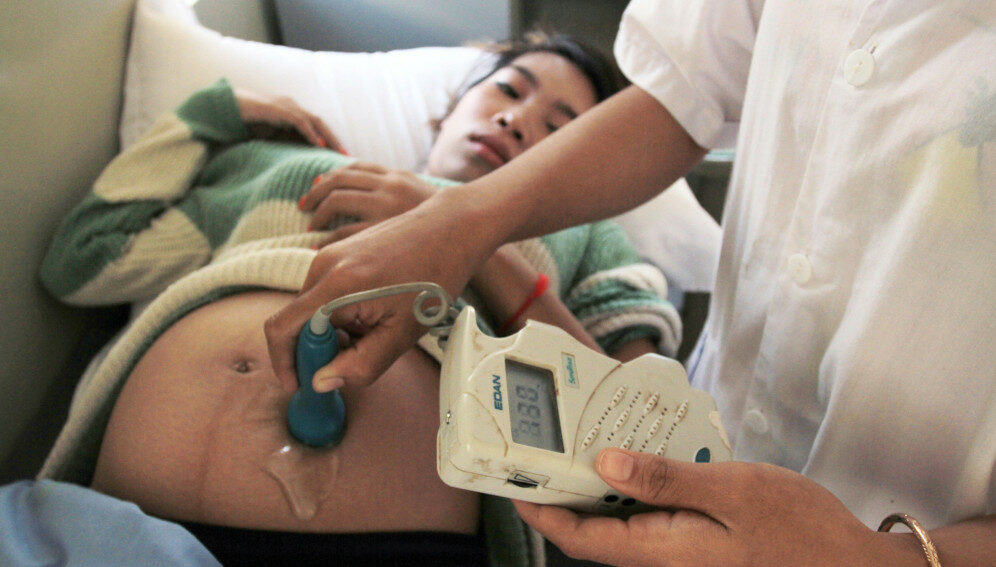
[NEW YORK] A single oral dose of the antibiotic azithromycin given during labour can dramatically reduce the risk of sepsis and death among women following childbirth, according to a large multi-country clinical trial, as a UN report warns of “stagnation” in maternal health.
Sepsis is a life-threatening condition which occurs when the body’s immune system overreacts to infections, damaging tissues and organs. It is a leading cause of maternal and newborn deaths worldwide, especially in low- and middle-income countries.
According to a report released by UN agencies today (23 February), 287,000 women died during pregnancy or childbirth in 2020 – the equivalent of one death every two minutes. It said that progress towards global goals on reducing maternal deaths had, at best, stalled in recent years.
“We are conducting studies on azithromycin resistance to make sure the intervention is also safe in addition to being effective in reducing infections”
Waldemar Carlo, co-director of the neonatology department at the University of Alabama’s Heersink School of Medicine
Researchers behind the azithromycin trials say preventative administration of the antibiotic during labour could not only stop hundreds of thousands of deaths from sepsis, but also ward off other infections after childbirth.
Waldemar Carlo, co-director of the neonatology department at the University of Alabama’s Heersink School of Medicine and one of the lead authors of the study, said the drug was chosen because “it covers many organisms that can be important and common pathogens in maternal infections”.
More than 29,000 pregnant women were enrolled in the trials in seven low- and middle-income countries: Bangladesh, the Democratic Republic of Congo, Guatemala, India, Kenya, Pakistan and Zambia. They were randomly assigned to receive either a two-gram dose of oral azithromycin or a placebo during labour.
Azithromycin, an inexpensive antibiotic effective against a broad range of bacteria, is known to reduce maternal infection when given intravenously during Caesarean delivery. Three smaller trials in the United States and Africa had also shown the potential to reduce maternal infections during vaginal births, says Carlo.
According to the study, published in the New England Journal of Medicine, only 1.6 per cent (227) of the women who received azithromycin developed sepsis or died within six weeks after delivery, compared to 2.4 per cent (344) of those who received the placebo.
Additionally, women who received azithromycin were less likely to develop endometritis, an infection of the lining of the womb, and other infections. They also had fewer hospital readmissions and unscheduled healthcare visits, compared to the placebo group.
“We are conducting studies on azithromycin resistance to make sure the intervention is also safe in addition to being effective in reducing infections,” Carlo added.
Administration of azithromycin did not reduce the risk of stillbirth, newborn sepsis, or newborn death, the research concluded.
The trial, called A-PLUS, was co-funded by the Bill & Melinda Gates Foundation and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), part of the US National Institutes of Health. The NICHD’s Global Network for Women’s and Children’s Health Research conducted the study between September 2020 and August 2022.
According to the researchers, A-PLUS was originally designed to enroll up to 34,000 women. However, based on a recommendation from the study’s independent data and safety monitoring committee, it was stopped early due to the clear maternal benefit of azithromycin.
Steven Simpson, head of the Sepsis Alliance and a professor in the Pulmonary Clinical Care and Sleep Medicine division at the University of Kansas, US, believes the findings could be life-saving.
According to the Global Maternal Sepsis study, 15 women per 1,000 live births in low- and middle-income countries have an infection which results in or contributes to death or life-threatening complications during their hospitalisation, based on data from 2017.
“There are 140 million births per year. More than half occur in Asia,” Simpson tells SciDev.Net. He calculates that the 1.68 million sepsis deaths per year could be reduced to 1.12 million, going by the results of the trial. “Approximately 560,000 women could avoid sepsis or death annually in Asia alone, if every mother received this treatment,” he says.
“Giving children a better chance to have a mother, and especially at relatively low cost, should be an important global initiative,” he adds.
This piece was produced by SciDev.Net’s Global desk.


