By: Jan Piotrowski
Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
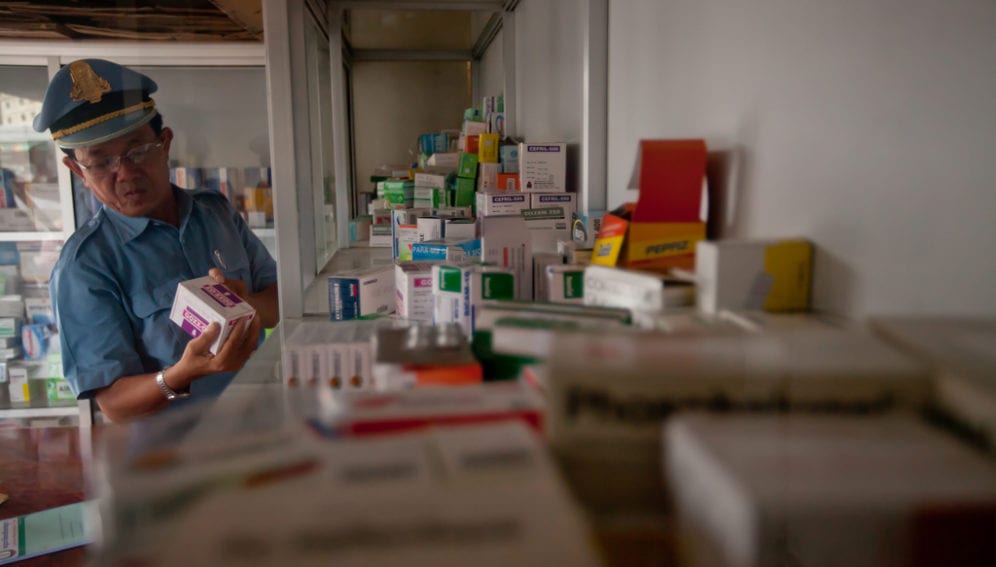
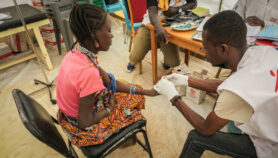
Despite the potential harm to patients from substandard drugs, over 60 per cent of malaria-endemic countries have no information on the quality of medicines used within their borders, according to the review of drug analyses, published in Malaria Journal this month (8 April).
The expense of building the necessary infrastructure of laboratories and regulatory bodies to monitor medicine quality means the issue is “barely mentioned” by the global health community, says Paul Newton, study co-author and a researcher at the University of Oxford’s Centre for Tropical Medicine, United Kingdom.
He says it is vital to check the quality of the actual drugs distributed. “After spending huge amounts of human and financial resources on developing new drugs and treatment regimes, it is a very illogical system where we neglect the quality of the final intervention,” he tells SciDev.Net.
As well as risking patients’ health and increasing the disease’s economic burden, medicines without the correct balance of active ingredients could increase the incidence of drug–resistant malaria, the report finds. This is because exposing pathogens to levels of a drug that are too low to kill them effectively increases the risk that survivors will become resistant and pass the trait to future generations.
“[Falsified or substandard medicines] are very likely to contribute to disastrous antimalarial artemisinin resistance, increasing mortality and morbidity and risking the loss of these vital medicines for malaria control,” the report says.
The paper, which systematically reviews more than 9,000 analyses of antimalarial drugs since 1946 in countries mainly in Africa, Asia and South America, finds that 30 per cent of samples failed quality tests. Counterfeit medicines could sometimes be identified by their fake packing, the report says, though chemical analyses — which sometimes revealed the drug’s active ingredient to be absent or present only at low levels — are also necessary, because official packaging for comparison is not always available in the developing world.
Of the drugs that failed tests, more than 39 per cent were classified as fraudulent copies, 2.3 per cent as unintentionally substandard and more than 58 per cent as poor quality for unclear reasons.
Yet only 41 of the 104 countries where malaria is endemic possess any publicly available data on medicine quality, the study says. Even when information exists, it is insufficient to fully understand the problem, it says.
For example, although malaria in Angola, the Democratic Republic of Congo and Gabon together account for an estimated 40 per cent of global disease burden, the paper says only one publically available analysis of the drugs in these countries is available.
Political will and foresight from governments and development donors is needed to see that investment in developing nations’ ability to police drug quality through governmental bodies, which is a low priority for donors and governments due to its expense, could save money, says Newton.
The WHO’s Rapid Alert System, which standardises and makes accessible information on substandard drugs that would otherwise remain hidden in government databases, helps, says Patricia Tabernero, a report co-author and the coordinator of the antimalarial quality group for the Worldwide Antimalarial Resistance Network.
But with only three laboratories in Sub-Saharan Africa and five in South-East Asia pre-qualified by the WHO as capable of accurately analysing the quality of antimalarials,
> Link to full paper in Malaria Journal
References
Malaria Journal doi: 10.1186/1475-2875-13-139 (2014)