By: Andrea Rinaldi
Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
In the last months of West Africa’s outbreak of Ebola, as the disease was fading away at the end of a two-year epidemic that took a devastating toll, biochemist Sterghios Moschos was putting the finishing touches on a new diagnostic test he hoped would help spot infections faster.
The assay, named EbolaCheck, was designed to detect the Ebola virus quickly and without sophisticated equipment, making it easy to deploy in the field or at airports.
As it turned out, the test was ready too late to test on the ground during the crisis. But confident it will perform well under the challenging conditions of working in many developing countries, Moschos is looking ahead to how it could help in the next emergency — and beyond Ebola.
“The principle of the assay can be applied to other viral diseases, which means that field testing can start soon for things like dengue virus, Zika virus, yellow fever, or West Nile virus — diseases much more common than Ebola,” he explains.
The test, which is based on technology invented by biotech company BioGene, has already been adapted to detect these viruses, and a field test is due to begin in January next year.
“Our method is mobile, requires a minimal power supply (the machine runs off a car cigarette lighter), needs a blood sample 700 times smaller than other methods, costs 10 times less, and gives a result in as little as 70 minutes, where it used to take up to 8 hours.”
Sterghios Moschos
Moschos and his team, based at Northumbria University in Newcastle, UK, started working on the diagnostic tool in 2014 — in the early stages of the Ebola haemorrhagic fever epidemic in West Africa — with help from a grant from the Research for Health in Humanitarian Crises (R2HC) Programme run by the NGO Elrha, which focuses on supporting research in emergencies.
Over the next two years, the epidemic would sweep across major urban and rural areas in the region, infecting about 28600 people in Guinea, Liberia and Sierra Leone, and resulting in over 11,000 deaths, according to the WHO. It is by far the worst epidemic since the appearance of the virus in 1976.
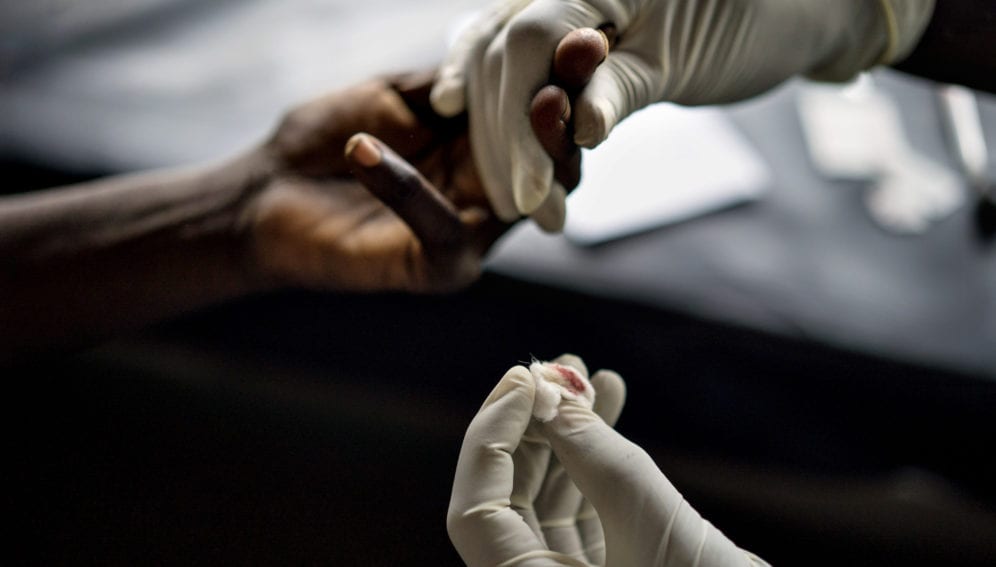
Ebola can spread quickly through person-to-person contacts, both in the community and in clinics. This makes diagnosis at the point-of-care pivotal in order to isolate infected people and limit transmission.
But picking up the infection is far from straight-forward: early symptoms, such as fever and weakness, are not specific to this infection, and are often seen in patients with other diseases that are more common in the same area — typhoid fever, for example, or malaria.
What’s new?
The science of EbolaCheck isn’t new: the test detects viral genetic material in fresh blood using reverse transcription polymerase chain reaction (RT-qPCR). Automated or semi-automated nucleic acid tests are recommended by the WHO for routine diagnosis of Ebola, and rapid tests based on the detection of Ebola virus proteins are also available, but usually less accurate than those detecting genetic material.
So, what makes the new test innovative?
“Our method is mobile, requires a minimal power supply (the machine runs off a car cigarette lighter), needs a blood sample 700 times smaller than other methods, costs 10 times less, and gives a result in as little as 70 minutes, where it used to take up to 8 hours,” explains Moschos.
The procedure involves withdrawing five microliters of blood from the patient’s finger using a sheathed lancet and capillary. The blood sample is then dispensed into a reaction chamber (‘puck’) that contains freeze-dried (lyophilised) reagents, and the puck is placed into a portable device for analysis.
“The gold standard test is a complex manual procedure that needs expensive equipment, a dedicated laboratory, and highly trained staff to carry it out,” adds Moschos. “We have shown that a great deal of this complexity can be eliminated using simple but precise temperature control, dyes that work in blood and fairly cheap parts”.
It’s thanks to those features that the new test can be done by staff with little training, wherever a patient might need it. Apart from the advantage of speed, it cuts the costs of diagnosis by a factor of ten, according to Moschos.
Armand Sprecher, a public health specialist and expert in Ebola management with the medical charity Doctors without Borders (Médecins Sans Frontières), whose staff were heavily involved in the emergency response in West Africa, points to the risks for health workers using any diagnostic tool, including EbolaCheck.
“Overall, the technology is interesting, although some caution with sample handling would still be required, as drawing blood from an Ebola suspect is never zero risk,” Sprecher points out. EbolaCheck eliminates a step in the lab testing process, which is the preparation of blood samples, and this limits some of the risk, he explains.“It might reduce the need for field lab support from external experts in some settings,” says Sprecher. “That being said, diagnostics is never as straight forward as one would hope, and having experienced lab people by our sides helped resolve some odd results we obtained.”
“I don’t see this [test] as revolutionary,” he concludes, “but maybe useful and have [an] application”.
A step forward
As the other available diagnostic tests, EbolaCheck can only detect the virus when it reaches a high-enough concentration in the bloodstream and symptoms, mainly fever, start to appear. This means infections cannot be picked up before this point — and it is this that could potentially help to reduce the spread of the disease in the initial phases of an outbreak.
“In other diseases we know that sometimes the body starts to send chemical signals out before symptoms kick off,” says Moschos. “But we don’t know what these signals are for this particular virus.”
So detecting the genome of the Ebola virus is still the most reliable approach, Moschos explains, and “that always works when someone has symptoms”. If a test was available to pick up infections before symptoms appear, “it would only serve to quarantine people suspected of exposure separate from those testing positive”. Doing this for up to 21 days, which is the incubation time of the virus, would help prevent onward transmission, he says.
Daniel Bausch, Director of the UK Public Health Rapid Support Team that worked with the WHO on the frontline during the West Africa crisis, believes that EbolaCheck is promising for low-resource areas.
“But a word of caution before we can declare this technology the solution to all our diagnostic problems: many assays look quite promising in the controlled laboratory environments of Europe or North America, but have challenges performing under the more rigorous high temperature and humidity conditions in the field,” says Bausch.
This is, in fact, the next step for Moschos and his team, after having published their findings in Chemical Science in November this year.
“In January 2018, [it] will be field tested by BioGene in rural areas in Brazil with over 400 patients to see how it performs ‘in the wild’,” says Moschos. “This is a great way to test potential challenges like humidity and temperature, and the simplicity of the operating procedure.”