12/02/21
WHO panel approves Oxford shot earmarked for COVAX
By: Esther Nakkazi
Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
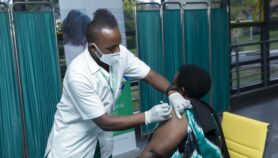
The World Health Organization expert group on immunisation has recommended the use of the Oxford/AstraZeneca vaccine against COVID-19, including in countries where the South Africa variant has been found.
Alejandro Cravioto, chairman of the Strategic Advisory Group of Experts (SAGE) on immunisation, told a press briefing on Wednesday there was “no reason not to recommend the use of the vaccine even in countries that have the variant”.
An interim recommendation issued by SAGE the same day said: “Countries should conduct a benefit-risk assessment according to the local epidemiological situation including the extent of circulating virus variants.”
“We should encourage countries to use this vaccine. Having reported one or two cases in the country doesn’t mean that variant is dominant,”
John Nkengasong, director, Africa Centres for Disease Control and Prevention
The Africa Centres for Disease Control and Prevention also supported the rollout of the AstraZeneca vaccine in all countries. Director John Nkengasong said: “We should encourage countries to use this vaccine. Having reported one or two cases in the country doesn’t mean that variant is dominant.”
The WHO expert panel reviewed the vaccine amid reports of lower efficacy against the B.1.351 strain of SARS-CoV-2, which was identified in South Africa and has spread to at least 30 other countries, including Kenya, Botswana, Mozambique and Bangladesh. Questions have also been raised over the vaccine’s efficacy in people aged over 65.
The AZD1222 shot developed by the University of Oxford and pharmaceutical giant AstraZeneca makes up the bulk of doses secured by the COVAX facility, which aims to ensure equitable access to COVID-19 vaccines.
South Africa suspended the rollout of the vaccine after researchers at the University of the Witwatersrand in Johannesburg released early trial data from a study of 18 to 65-year-olds showing no measurable effect on mild or moderate disease caused by the B.1.351 variant following the administration of two doses.
The Bill & Melinda Gates Foundation said the study’s findings were “deeply disappointing”, but credited the South African researchers for generating valuable new knowledge that would enable more targeted interventions and inform decision-making about vaccine rollouts.
Primary analysis of data from Phase III trials has so far shown — in the context of viral settings without the South African variant — that the Oxford/AstraZeneca vaccine offers protection against severe disease, hospitalisation and death.
Some European countries have limited the use of the vaccine among older age groups while others have ruled out its use entirely, because of a lack of trial data on people aged over 65.
However, the WHO expert panel recommended the use of the AstraZeneca product even among people aged 65 and above. The interim statement says more precise efficacy estimates for this age group are expected soon, from ongoing trials and vaccine effectiveness studies in countries using the vaccine.
The WHO is expected to give the vaccine an emergency use listing later this month.
James Fulker, a spokesman for GAVI, the Vaccine Alliance, told Scidev.Net: “The AstraZeneca vaccine is a safe and effective vaccine, including for over 65s. We do not have evidence that it is not effective against the B.1.351 variant. The B.1.351 variant is not dominant in most of the world.”
He said plans for the rollout of the vaccine would continue pending the WHO listing, with “significant volumes” available from this month.
COVAX, coordinated by GAVI, the WHO and the Coalition for Epidemic Preparedness Innovations (CEPI), had announced an initial rollout of 336 million doses of AstraZeneca vaccines starting February.
The WHO SAGE interim recommendations also okayed vaccination for people with co-morbidities and those previously infected with SARS-CoV-2. However, they said there was limited data for children and adolescents below the age of 18 years, pregnant or lactating women, or people living with HIV.
It cautioned against vaccinations for patients with current acute COVID-19 and said co-administration with other vaccines should be done with a minimum interval of 14 days, subject to further data.
A joint statement by UNICEF executive director Henrietta Fore and WHO director-general Tedros Adhanom Ghebreyesus called on leaders to look beyond their borders and employ a vaccine strategy that could end the pandemic and limit variants.
“COVAX participating countries are preparing to receive and use vaccines. Health workers have been trained, cold chain systems primed. What’s missing is the equitable supply of vaccines,” they said in a statement.
More than 90 per cent of countries now rolling out vaccines are wealthy, while almost 130 countries, with a total of 2.5 billion inhabitants, have yet to administer a single dose.
Mark Suzman, CEO of the Bill & Melinda Gates Foundation, said: “We will use our funding commitments of more than US$1.75 billion to help accelerate the development and distribution of vaccines that are optimised for lower- and middle-income countries and are effective against the variants.”
This article was originally published on SciDev.Net’s Global edition.