Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
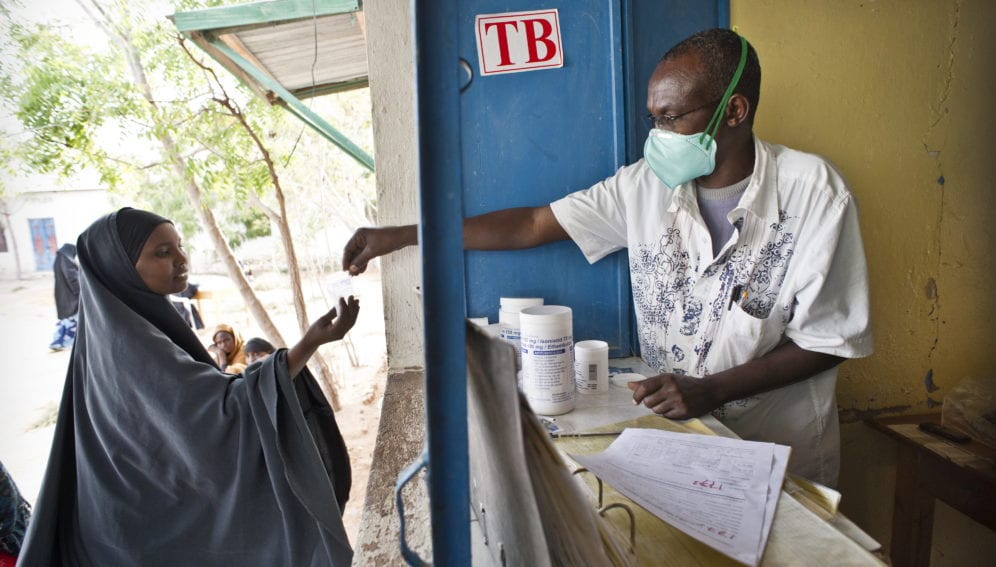
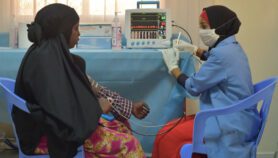
Co-administering a drug for treating HIV called dolutegravir with two common TB medicines can safely shorten TB treatment, a study says.
The WHO estimates that those living with HIV are 20 to 30 times more likely to develop active TB, with a third of all HIV deaths every year being attributed to TB. According to WHO guidelines, longer treatment of drug-resistant TB requires drugs to be administered for about 20 months whereas shorter treatment has a duration of nine to 12 months.
But a study conducted in South Africa from January 2018 to February 2019 by the Aurum Institute and partners shows that administering dolutegravir for eight weeks and subsequently adding three-month of two first-line TB medicines — rifapentine and isoniazid (3HP) — can safely be used to treat TB in people with HIV.
“The plan is to scale up 3HP for people living with HIV and all household contacts.”
Gavin Churchyard, Aurum Institute
“We wished to show that 3HP with dolutegravir in people living with HIV was safe. The 3HP will be very useful for preventing TB in people living with HIV,” Gavin Churchyard tells SciDev.Net.
Churchyard, group CEO of South Africa-based Aurum Institute and co-principal investigator of the study, adds that the research finding paves the way for scaling up the 3HP regimen in 12 high-burden TB countries: Brazil, Cambodia, Ethiopia, Ghana, India, Indonesia, Kenya, Malawi, Mozambique, South Africa, Tanzania and Zimbabwe.
Researchers presented the findings of the study — which involved 60 HIV-positive adults in South Africa and has not yet been published in a journal — at the Conference on Retroviruses and Opportunistic Infections in Seattle, United States last week (6 March).
According to the researchers, after completing treatment, the participants were followed for four more weeks. Co-administration of dolutegravir, rifapentine and isoniazid was well-tolerated, with no serious adverse drug reactions, the researchers add.
“The plan is to scale up 3HP for people living with HIV and all household contacts, particularly children under five years, in all high burden countries.” Churchyard explains, adding that his team is seeking funding to help make the drugs accessible to poor people living with HIV and with TB.
Kizito Lubano, a clinical researcher at the Kenya Medical Research Institute and an honorary lecturer at Kenya-based University of Nairobi School of Medicine, agrees with the study’s findings but says that the small sample size of 60 is not enough for making recommendations for large-scale use.
“There is a need for comprehensive multi-centre phases three and four trials with larger sample sizes to have sufficient confidence to recommend for routine use,” he tells SciDev.Net.He adds that any simplification of treatment options leading to shorter duration and low number of medicines is a desirable goal of any disease treatment and control programme.
“The current treatments are the best based on scientific advancement and knowledge of HIV/AIDS and TB,” says Lubano. “However, there is a need for continuous improvement as knowledge increases.”
This piece was produced by SciDev.Net’s Sub-Saharan Africa English desk.