Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
Many challenges lie ahead for the Cartagena protocol to be effective, Maria Elena Hurtado reports.
[SANTIAGO] Ten years after the Cartagena Protocol on Biosafety entered into force to detail the safe handling, transfer and use of living genetically modified organisms, vast majority of its 164 signatories have not fully implemented it.
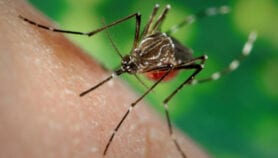
Yet in recent years, researchers have started producing genetically modified fish in Panama for human consumption, and releasing genetically modified mosquitoes into the wild in Brazil and elsewhere to try and prevent dengue fever, sometimes with unclear safety and regulatory oversight.
The protocol commits signatory countries to appoint a national authority to administer the protocol, to create national biosafety frameworks and regulations, and to build capacity for risk assessment and the safe handling and transport of living modified organisms (LMOs).
Fifty-two countries have domestic regulations fully in place, 75 have one or more biosafety laws and almost all of them have national authorities for administrating it, reports an article published in the anniversary edition of the protocol's newsletter.
"But still there is a long way to go to make sure the national biosafety rules and regulations in place are workable and countries have the necessary capacity to enforce them," Braulio Ferreira de Souza Dias, executive secretary to the Secretariat of the Convention on Biological Diversity, tells SciDev.Net.
"For example, the effectiveness of biosafety regulations will be minimal unless countries have the necessary tools to detect and identify LMOs."
Challenges ahead
And for the protocol to be fully effective, "we need to work towards achieving its universal membership", said de Souza Dias, in a press release published earlier this month (10 September). "I call upon all countries that have not yet done so to fast track their national processes to ratify or accede to the Cartagena protocol … as soon as possible."
“The absence of legal certainty in many countries has been commonly regarded as one of the most serious stumbling blocks in the path of biodiscovery.”
Braulio Ferreira de Souza Dia, Convention on Biological Diversity
Also, the speed of implementation has decreased over the last three years, Stefan Jungcurt of the Canada-based International Institute on Sustainable Development, tells SciDev.Net.
"As more and more countries approve LMOs for cultivation and import, the political priority given to biosafety is diminishing, which translates into a lack of financial and other support to implement the protocol on a national level," he says.
Lorem ipsum et optaque ventint fugit hit quam in sanducia verorest.In his view, a key pending issue is approving UN guidelines for risk assessment and risk management. Claudio Chiarolla, director of biodiversity governance at the Institute for Sustainable Development and International Relations, a non-profit policy research institute based in France, agrees.
Both Jungcurt and Chiarolla tell SciDev.Net that one of the most important issues will be to determine the protocol's scope regarding the potential socioeconomic impact that LMOs pose for the sustainable use and conservation of biodiversity, and what appropriate action can be taken.
"The protocol will also have to deal with other types of LMOs such as genetically modified mosquitoes, aquatic species, microorganisms or products of synthetic biology, which are different to LMO crops," Jungcurt adds.
A new international treaty was adopted at a 2010 meeting in Japan, called the Nagoya – Kuala Lumpur Supplementary Protocol on Liability and Redress to the Cartagena Protocol on Biosafety.
This treaty deals with potential damages resulting from the export and import of LMOs.
Jungcurt says: "The most immediate challenge here is achieving its coming into force and its national implementation, as well as establishing rules and procedures on who is liable if damage occurs during transboundary movements".
A fair share
De Souza Dias told a meeting in Denmark this month (4 September) that the Nagoya protocol "put an end to the mistrust among industry, indigenous and local communities over the equitable sharing of benefits".
"The absence of legal certainty in many countries has been commonly regarded as one of the most serious stumbling blocks in the path of biodiscovery," he adds. "The Nagoya protocol seeks to address this concern."
He recognises the high stakes involved for different groups. "Over the last two decades, the issue of free trade in LMOs on the one hand, and biosafety on the other, have led to heated debates and, at times, to tense legal disputes."
But he believes industry has been making efforts to work with governments within the process of the biosafety protocol.
"The participation of representatives of industry and civil society in the Cartagena protocol process has been helpful to maintain transparency and balance in taking decisions," he says.