By: Alex Namwena
Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
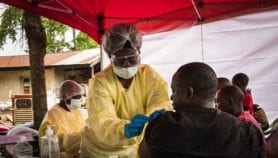
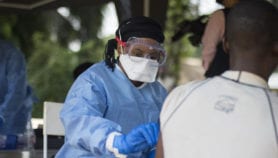
An experimental Ebola vaccine is being administered for the first time, in an Ebola outbreak in the Democratic Republic of Congo (DRC). No vaccine has yet been licensed to control the Ebola virus. However, in order to contain the epidemic that has been raging for nearly two weeks in the north-west of the DRC, the World Health Organization (WHO), on a “compassionate” basis and following approval from the Congolese authorities, has decided to vaccinate about 600 people.
"The vaccination campaign is first and foremost for medical staff and people who have been in contact with laboratory-confirmed cases of Ebola," says Jean-Jacques Muyembe, a virologist, an expert on Ebola and director of the National Institute for Biomedical Research (INRB) in Kinshasa.
The DRC announced a new Ebola outbreak earlier this month (May 8). The virus appeared in Bikoro, a rural enclave in the Equateur province. A case has also been confirmed in Mbandaka, a city of about one million inhabitants, the largest agglomeration in northwestern DRC.
A report published on May 20 by the WHO, mentions27 deaths.
The vaccine, rVSV-ZEBOV (Recombinant vesicular stomatitis virus-Zaire Ebola virus), was developed by the National Microbiology Laboratory of Canada. It has been shown to be effective in limited trials in West Africa, where the largest Ebola outbreak killed 11,300 people between 2014 and 2016.
According to the Congolese Health minister, Oly Ilunga, "this is a vaccine that has proven effective in West Africa, and none of the people who were vaccinated during the incubation period developed the disease."
Of the five species of Ebola virus, rVSV-ZEBOV protects against a strain known as Zaïre (the former name of the Democratic Republic of Congo), which most commonly infects people. The vaccine works by tricking the body into believing that it has been infected with the virus, which triggers an immune response.
When it was developed in 2003, rVSV-ZEBOV was found to be 100 per cent effective in monkeys.
But the pharmaceutical industry had shown little interest in developing it until the Ebola outbreak that began in West Africa in 2014. That year, German pharmaceutical company Merck acquired production rights.
According to the Congolese government, "partners have promised 30,000 doses of the vaccine". But only 5,400 doses have been delivered so far.
A major challenge will be adequate refrigeration. The vaccines need to be stored within a restricted temperature range (minus 60 to minus 80 degrees Celsius) from the manufacturing stage to the point of administration. This could prove challenging in a location like Bikoro, which sorely lacks electricity.

According to the WHO, health facility managers will monitor patients for 84 days after vaccination. In addition to vaccination, an array of measures has been put in place.
Experts believe that if rVSV-ZEBOV proves safe and effective, it could change the way health officials respond to the disease and can help make sure that it never reaches epidemic proportions again.
Not an emergency
According to the WHO, the risk of the current outbreak spreading is "high", but the epidemic does not constitute a global health emergency — so there is no need to limit people's ability to travel freely at this stage, it has said.
However, controls at airports, ports and borders have been stepped up to detect suspected Ebola cases.
Ebola is transmitted through contact with infected body fluids. Authorities and NGOs are therefore raising awareness about the need for strict hygiene measures: washing hands with soap, avoiding unnecessary bodily contacts, prohibiting the collection of dead animals in the bush and prohibiting unsafe traditional burial rites.
"Funerals actually promote the spread of the disease; there are traditions in our country that require the body to be washed and touched. This is now forbidden during this period," Ilunga says.This is the ninth Ebola outbreak in DRC. Since its discovery in 1976, the virus has resurfaced endemically in the country. "There is resurgence of the disease because until now, we do not know which animal is the virus's natural reservoir or which insect transmits it," says Muyembe.
Researchers thought that bats were the initial vector of the disease. But "following analysis of more than 10,000 samples, this could not be scientifically confirmed", the researcher adds.
Congolese researchers are also trying to develop a cure for Ebola, says Oly Ilunga: "A monoclonal antibody has been isolated from one of the survivors of the Kikwit epidemic. This antibody is in fact the next generation of therapeutic molecules against Ebola. We will soon start clinical trials."
This article was produced by SciDev.Net’s Sub-Saharan Africa French desk.