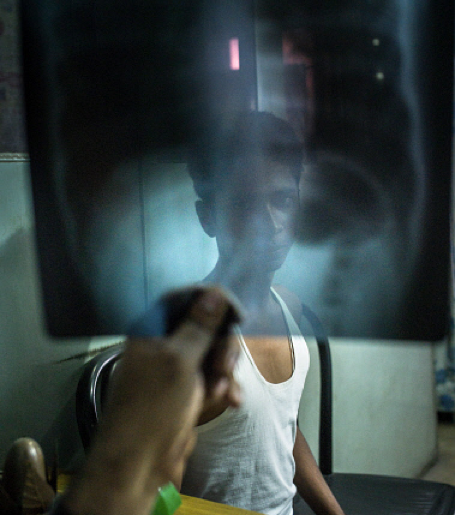By: Fatima Arkin
Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
[MANILA] As nations observe World Tuberculosis Day on 24 March on the theme “unite to end TB”, a new public-private partnership is widening access to a drug to fight one of the world’s top infectious killers.
Japan-based pharmaceutical company Otsuka is working with the WHO-led Stop TB Partnership’s Global Drug Facility to help more than 100 low- and middle-income countries gain access to delamanid, one of only two medications developed in the past 40 years to fight multidrug resistant tuberculosis (MDR-TB).
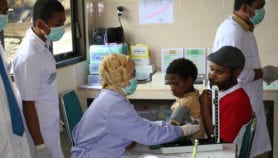
Under the new partnership, countries in South-East Asia and the Pacific can purchase delamanid for US$1,700 per six-month treatment course per patient, a 95 per cent reduction from the medication’s publicly listed price in Europe. The Stop TB Partnership secretariat and its partners will provide technical assistance and other services to ensure financing and swift incorporation of delamanid into national TB programmes.
“Patients have waited far too long for new treatment innovations and we hope this partnership will help turn the tide against this deadly disease in South-East Asia,” Masuhiro Yoshitake, managing director of Otsuka and TB global project leader, tells SciDev.Net.
But Grania Brigden, TB advisor at the humanitarian organisation Médecins Sans Frontières (MSF or Doctors Without Borders), says that although the reduced price for delamanid does not appear overly high, it is going to be added into a treatment regimen that already costs US$1,800-$4,600 while a number of high burden countries are transitioning out of global fund support of their TB programmes.
“We worry that this price will be prohibitive for countries to cover themselves, which will slow down the much needed scale-up of this new drug,” Brigden tells SciDev.Net.
According to the WHO’s 2015 world tuberculosis report, in 2014, there were an estimated 480,000 new cases of MDR-TB worldwide and roughly 190,000 deaths resulting from MDR-TB. High burden countries in South-East Asia include Myanmar (5 per cent), Vietnam (4 per cent), Philippines (2 per cent) and Indonesia (1.9 per cent).
Globally, only half of patients on MDR-TB treatment were successfully treated, largely due to high rates of mortality and loss to follow-up, says the report.
“Early results show that delamanid has real potential to radically improve the outcome for patients with drug resistant forms of TB,” says Brigden. “The WHO recommendations are broader for delamanid than they are for bedaquiline, the other new drug, and it looks to be a better drug to use in patients co-infected with HIV.”
In 2015, delamanid was put on the WHO’s list of essential medicines, the most important medications needed in a basic healthcare system. But since delamanid was approved in Europe in April 2014, there have been less than 200 patients globally who have had access to it. A report from the MSF shows that up to two-thirds of patients with MDR-TB may be eligible for delamanid using WHO criteria.
This piece was produced by SciDev.Net’s South-East Asia & Pacific desk.