By: T.V. Padma
Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
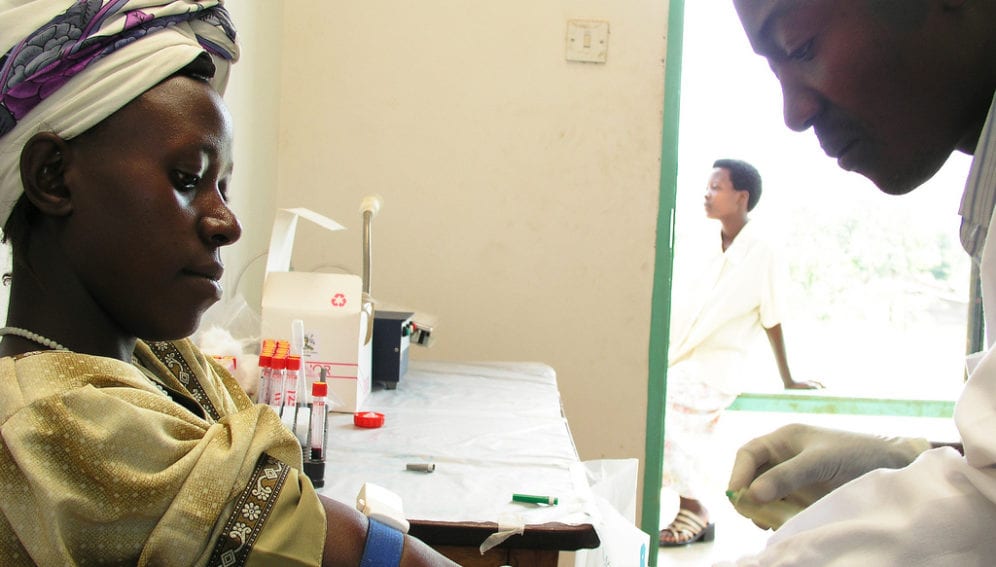
[NEW DELHI] High costs and logistic hurdles have delayed the rollout of point-of-care (POC) diagnostic tests for HIV in resource-poor countries where they are urgently needed, experts say.
Affordable HIV diagnostics, including POC tests, is a global concern. There are two types of HIV tests: One counts the number of a type of immune cells, called CD4 cells, to measure the health of a patient's immune system, and the second counts the number of virus copies or 'viral load' in the blood.
The international medical charity Medicins Sans Frontieres (MSF) is expected to release this year a report on the availability of viral load testing, including POC tests, in six countries.
“The growing numbers of high-quality POC diagnostic technologies may alleviate the challenges seen in the conventional laboratory network and provide access to testing for those without (access),” Lara Vojnov, diagnostic scientist, Clinton Health Access Initiative, Liberia, told a symposium on HIV science that ended early February in Chennai.
At US$ 11 per test, CD4 POC tests are considered unaffordable. In many countries a few large centralised clinics cater to CD4 testing demand, whereas POC testing implies decentralised services in smaller clinics.
Current CD4 cell-measuring techniques require a costly instrument and trained personnel. Patients need to revisit the clinic for results, leading to dropouts, or delays in initiating treatment.
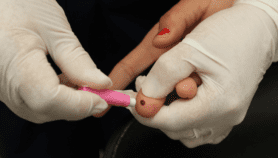
The new CD4 POC test can be done within 10 minutes of collecting a blood sample and gives results within 30—40 minutes, obviating the need for patients to come back for test result confirmation.
A self-testing kiosk developed at the Johns Hopkins University offers a CD4 test using an oral swab; while a disposable CD4 cell test using finger-prick blood has been developed at the Burnet Institute, Melbourne. Other POC detection methods include a gene amplification test developed at the University of Cambridge and tested by MSF in Malawi and Uganda.
POC diagnostic technologies have positively impacted patient retention and reduced the time to start anti-HIV treatment, Vojnov said.
MSF, which is evaluating both kinds of tests in resource-poor settings, says POC viral load tests are the need of the hour as they detect infections and possible treatment issues early.
“It is important to catch high viral load in time as it helps check patients' adherence to treatment and detects emerging drug resistance that warrants a switch to newer drugs,” Leena Menghaney, India coordinator of MSF's Access Campaign, tells SciDev.Net.
A preliminary briefing paper released by MSF in December 2013 says that there is room to further cut the costs of both kinds of tests. It says the largest price factor is the cost of reagents and other non-device products needed to run the test — more than half and up to 83 per cent of overall costs.
The price per test also depends on the volume of tests run on each instrument.
MSF also found "significant" costs associated with intellectual property rights. Most require portable cartridges to run the tests; and the manufacturing costs of an integrated and complex cartridge is almost twice as high as the cost of lab-based tests.
The briefing says prices could drop through negotiation, pooling tests and using existing networks to deliver samples and results from testing.