By: Claudia Caruana
Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
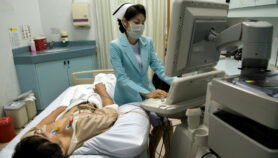
[NEW YORK] A portable 3-D scanning device developed by Sri Lankan and US researchers can quickly measure limb enlargement of patients with the disfiguring condition elephantiasis that resulted from lymphatic filariasis infection.
Reporting on the device this month (16 October) in the American Journal of Tropical Medicine and Hygiene, the researchers say it consists of an infrared sensor mounted on an iPad that produces highly accurate 3-D reconstructions of the legs — the limbs most commonly affected by lymphedema or fluid accumulation from blocked lymphatic vessels.
“The scanner produced highly accurate results in only a fraction of the time of the other tests”
Philip Budge, Washington University in St. Louis
Philip Budge, an author of the report and professor of medicine in the division of infectious diseases at Washington University, St. Louis, says healthcare workers assess the severity of lymphedema by using a tape measure or by relying on water displacement by the affected limb. Often, this is impractical for leg measurements.
Originally developed to measure lymphedema in cancer patients following surgical removal of the lymph nodes, the new device has been adapted for those afflicted by elephantiasis caused by lymphatic filariasis infection.
Lymphatic filariasis is a mosquito-borne disease that often leaves its victims with disabling hydrocele (accumulation of fluid in the scrotum), lymphedema or elephantiasis after the infection has cleared. According to the WHO, infections typically occur during childhood but may go unnoticed, leading to a permanently impaired lymphatic system.
Budge and fellow Washington University researcher, Ramakrishna Rao, tested the device on 52 patients with varying stages of lymphedema at a clinic in Galle, Sri Lanka, managed by Channa Yahathugoda, a co-author of the report.
“The most encouraging news is that the scanner produced highly accurate results in only a fraction of the time of the other tests,” Budge says. “To our knowledge, this is the first time that infrared 3-D scanning technology has been used on filarial lymphedema patients.”
Budge stresses that lymphedema is not the same as an infection with the parasite that causes lymphatic filariasis. “Sri Lanka has nearly eliminated transmission of the parasite, but many people who were previously infected still have lymphedema which doesn’t go away even after treatment to clear the parasites.”
Mike Weiler, the chief of Lympha Tech which is developing the first commercial version of the device, says it should be ready for limited release during the first quarter of 2018 and open availability in the second half of 2018. “The fact that it's a mobile-based platform allows it to be affordable for nearly every environment,” he tells SciDev.Net.Achim Hoerauf, director of the Institute of Medical Microbiology, Immunology and Parasitology, Bonn, Germany, says the new device will be useful in lymphedema clinics and in lymphatic filariasis control programmes. It could, he says, “lead to early detection of limb abnormalities in people at risk for lymphedema and early morbidity management and prevention.”
This piece was produced by SciDev.Net’s Asia & Pacific desk.