12/11/19
New dengue vaccine effective in clinical trials

By: Neena Bhandari
Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
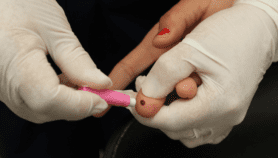
[SYDNEY] An experimental dengue vaccine has proved 80.2 per cent effective against virologically-confirmed dengue among children and teens aged 4—16 years in the 12 months after a second dose, according to results of phase 3 clinical trials.
“A vaccine with this kind of efficacy could have a substantial impact on public health,” says Derek Wallace, contributor to the trial results, published 6 November in The New England Journal of Medicine. According to the WHO, dengue is one of the top 10 threats to global health, infecting nearly 400 million people and killing up to 25,000 people worldwide annually. There is no specific treatment for the mosquito-borne viral disease, which causes flu-like symptoms, joint and muscle pain and, in severe cases, leads to haemorrhagic fever and death. It is now endemic in more than 100 countries, with Asia shouldering 70 per cent of the disease burden.
“A vaccine with this kind of efficacy could have a substantial impact on public health”
Derek Wallace, Takeda Pharmaceutical Company Limited
“Our dengue vaccine candidate, TAK-003, is based on a live-attenuated dengue serotype 2 virus (DENV-2), which provides the genetic ‘backbone’ for all four vaccine serotypes,” Wallace, head of the global dengue programme of the Takeda Pharmaceutical Company Limited, which developed the vaccine, tells SciDev.Net.
A preliminary analysis of TAK-003 shows different efficacy levels against the four strains of dengue. Vaccine efficacy was most effective for serotype 2 at 97.7 per cent, dropping to 73.7 per cent for serotype 1 and 62.6 per cent for serotype 3 and inconclusive for serotype 4. In participants who had confirmed dengue infections, the vaccine reduced the risk of hospitalisation by 95.4 per cent. In comparison, Sanofi Pasteur’s Dengvaxia (CYD-TDV), the world’s first dengue vaccine to be licensed, had 59.2 per cent efficacy in the first year of follow-up based on the combined results from two late stage trials.
Humberto Reynales, lead author of the study and executive director of The Caimed SAS Medical Care and Research Centre in Bogota (Colombia), tells SciDev.Net that the results “suggest that due to the onset of some protection after the first dose, the vaccine may be useful in the context of outbreak control or travel vaccination. “However, reported variation in serotype-specific efficacy needs careful consideration and more analysis. Dengue vaccination, where implemented, should be part of a comprehensive public health strategy.”
Dengue virus is transmitted by female mosquitoes mainly of the Aedes aegypti species and, to a lesser extent, the A. albopictus, a secondary dengue vector in Asia. Immunity to one strain of dengue virus serotype does not provide immunity against the others.
Dengvaxia became controversial after it was disclosed in 2017 that it could increase the risk of severe dengue and hospitalisation in children who had never been infected by dengue (seronegative). The Philippines, which had immunised more than a million children with Dengvaxia, ordered its withdrawal.
“The structure of our vaccine candidate, the unique immunogenicity profile, and the efficacy in participants regardless of previous dengue exposure, are reasons that we believe TAK-003 has a different safety and efficacy profile relative to the currently licensed dengue vaccine,” Wallace says. “Takeda’s dengue vaccine candidate was generally well tolerated, and no important safety risks have been observed to date.”
“In regard to serious adverse events, these occurred at similar rates in individuals who received TAK-003 and individuals who received placebo (3.1 per cent and 3.8 per cent). We are continuing to assess safety and efficacy over a total of four-and-a-half years,” Wallace adds.
Trials of the vaccine are currently being conducted in dengue-endemic countries in Asia (the Philippines, Sri Lanka and Thailand) and Latin America (Brazil, Colombia, Dominican Republic, Nicaragua and Panama).
Experts caution that long-term evaluation is needed to assess the safety of the vaccine. The final phase of the Tetravalent Immunisation against Dengue Efficacy Study trial will evaluate efficacy and long-term safety by following up on participants for an additional three years.
Cameron Webb, mosquito researcher at the New South Wales Health Pathology, Sydney, tells SciDev.Net, "There needs to be a high level of confidence that not only will a new vaccine protect the community but also that it won't make the problem worse. Long-term studies are required to ensure that there is no evidence that more severe dengue is occurring in those vaccinated.”This piece was produced by SciDev.Net’s Asia & Pacific desk.