Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
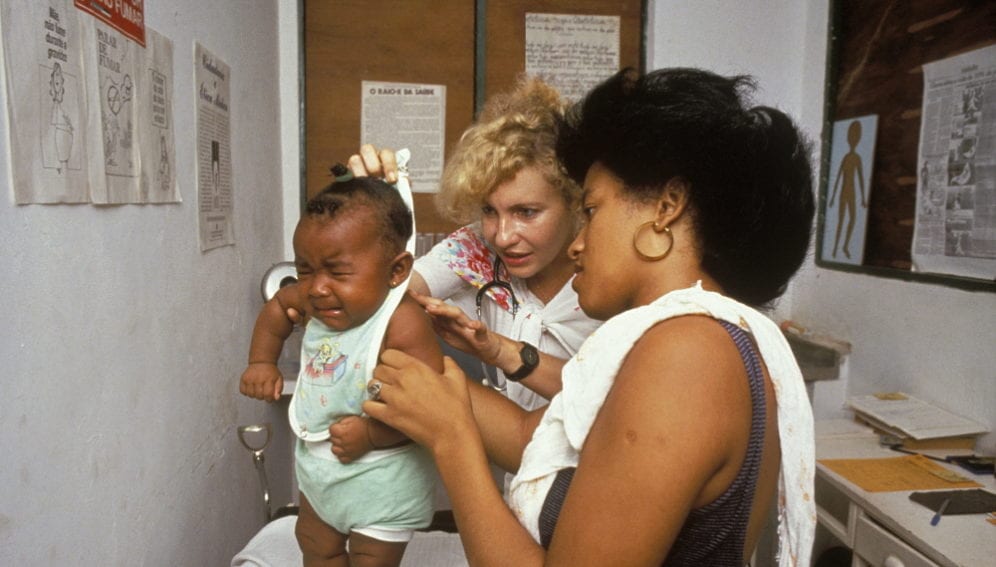
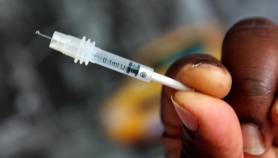
[SAO PAULO] A vaccine for parasitic intestinal worms has been shown to be safe in Brazilian clinical trials, according to its US developer.
The trials were carried out in the city of Belo Horizonte, and the rural settlement of Americaninhas in eastern Brazil where there is a high risk of hookworm infection.
Hookworm parasites infect more than 600 million people worldwide, attaching themselves to the intestines to feed on blood. Infection can lead to iron deficiency and capillary damage, and may retard children’s growth and mental development.
“A vaccine able to induce long-lasting immunity would provide a sustainable solution to the problem of hookworm.”
David Diemert, Sabin
Researchers from the Sabin Vaccine Institute Product Development Partnership found the vaccine was well tolerated by 102 healthy volunteers and blood tests showed that they developed an immune response. None of them had ever been exposed to hookworm before.
David Diemert, Sabin’s director of clinical trials, tells SciDev.Net: “A vaccine able to induce long-lasting immunity, which could be incorporated into existing vaccination programmes in countries like Brazil, would provide a sustainable solution to the problem of hookworm.”
The vaccine’s active ingredient is a protein from the adult Necator americanus hookworm, the species that most commonly infects people. When patients are exposed to a harmless form of the protein, they develop antibodies that recognise it. In theory these would then be able to recognise the proteins — and mobilise the immune system — if the person was subsequently infected with worms.
Researchers say the vaccine could be an alternative way to deal with repeat infections. Brazil’s public health system distributes single dose anti-hookworm drugs to local people for free, but they are often reinfected by contaminated water. In theory, the vaccine could treat existing infections and provide lasting immunity.
Although it raises hopes of better hookworm control, it will still be several years before the vaccine has undergone all the tests necessary to be approved for use, says Diemert. But he hopes to have a licensed vaccine by 2020.
The Sabin initiative is part of the wider HOOKVAC consortium that is developing the vaccine. Another trial is expected to start this month in Gabon, where three in ten people have hookworms.
Ronaldo Scholte, a senior consultant at the Brazilian Health Ministry, tells SciDev.Net that any future vaccine would be an important tool to control hookworm infections.
But he notes that “basic sanitation is as important as new drugs to treat waterborne diseases”.
Manoel Victor Lemos, an applied biologist at Sao Paulo State University, Brazil, says a vaccine would be the “most sustainable control of hookworm we have ever had”.