By: Fatima Arkin
Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
[MANILA] A potential malaria vaccine described as the “end game” for malaria got the much needed lift to speed up its development after the Japan-based Global Health Innovative Technology (GHIT) Fund announced last week (March 31) that it will provide financial support on the innovative malaria vaccine.
Rhoel Dinglasan, the recipient of the GHIT award, heads a team at the University of Florida in the United States which is working with the Japan-based biotech company CellFree Sciences to develop the vaccine that will block two strains of the disease, the deadly Plasmodium falciparum parasite and the more widespread P. vivax.
“Unless we have a way to both block transmission of the malaria parasite from human to mosquito, and target its two most destructive strains, we may never see its end.”
By BT Slingsby, CEO of GHIT Fund
GHIT calls the CellFree-UF proposal the “leading” mosquito-based transmission-blocking vaccines (TBV) candidate in which the vaccine is given to humans, but mosquitoes are the targets.
Several TBVs are currently being developed but the CellFree-UF TBV candidate is the only one that can potentially block both strains of the disease.
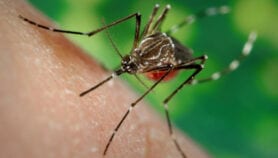
The vaccine candidate targets a protein in the mosquito’s gut called AnAPN1, which plays a crucial role in enabling the transmission of both types of malaria parasites from humans to mosquitoes. When the vaccine is given to humans it will provide them with protection that would subsequently interfere with the parasites’ movement into mosquitoes.
GHIT will invest about US$400,000 to develop the CellFree-UF vaccine candidate that is currently in the preclinical trial phase. GHIT is a public-private partnership between seven Japanese pharmaceutical companies, the Government of Japan, the Bill & Melinda Gates Foundation, the Wellcome Trust and the UN Development Programme.
“Malaria has taken its deadly toll on the world’s most vulnerable people for far too long, and unless we have a way to both block transmission of the malaria parasite from human to mosquito, and target its two most destructive strains, we may never see its end,” BT Slingsby, CEO of the GHIT Fund, tells SciDev.Net. “We believe this approach has an enormous role to play in finally eradicating malaria once and for all.”
According to the WHO, 1.4 billion people in South-East Asia are at risk of malaria and over 1,800 people died from the disease in 2011. An October 2015 article in the scientific journal Nature Communications reports that drug-resistant malaria parasites are quickly spreading in parts of South-East Asia and are capable of infecting the type of mosquito that is the main transmitter of malaria in Africa. In May 2015, the World Health Assembly vowed to eliminate malaria from 35 countries and reduce case incidence and mortality rates by 90 per cent worldwide.
Dinglasan says that if all goes according to plan, they can potentially reach the first phase of clinical trials in around four years. But he stresses that the vaccine is intended for the “end game” of malaria elimination and eradication. “So even if it is ready to go by 2025, for example, ideally it will be kept until the very end to wipe out the disease.”
This piece was produced by SciDev.Net’s South-East Asia & Pacific desk.