By: Claudia Caruana
Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
[NEW YORK] Researchers at the Boston-based Massachusetts Institute of Technology (MIT) have devised a capsule that allows staggered release of ivermectin, a broad anti-parasitic drug including against malaria parasites, obviating the need for daily doses and promising better patient compliance.
Giovani Traverso, a medical researcher affiliated with the Koch Institute for Integrative Cancer Research at MIT and Robert Langer, a chemical engineering professor at MIT, developed the capsule which contains a star-shaped drug release system.
“We applied this new technology to efforts that may lead to the elimination of malaria.”
Giovani Traverso, Koch Institute for Integrative Cancer Research at MIT
After ingestion, the capsule dissolves in the stomach to release the delivery system that dispenses the drug in measured doses for up to two weeks before finally dissolving and getting eliminated from the body. According to Traverso, the device is resistant to gastric acids and the star shape prevents it from blocking the normal function of the digestive system.
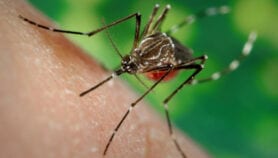
“We applied this new technology to efforts that may lead to the elimination of malaria,” said Traverso. “Specifically, we focused on ivermectin because it works against malaria parasites, and is also toxic to malaria-carrying mosquitos when they bite people with ivermectin in their system.”
Initial human trials with this delivery system are set to be conducted later this year by Lyndra, Inc., which is based in Watertown, Massachusetts, and has been developing long-acting oral drug delivery technologies. Langer is a co-founder of Lyndra.
The research for a better ivermectin delivery system was supported by a grant from the U.S. National Institute of Biomedical Imaging and Bioengineering Technology and its development in Langer’s laboratory had funding from the Bill and Melinda Gates Foundation.
The developers say the system can help do away with daily doses of ivermectin. Eliminating malaria — which kills more than half a million people annually and affects millions more – is a challenge partly because a large portion of the at-risk population lives in rural areas with limited access to health care, making drug compliance difficult.
“Any approach that reduces the frequency at which medications need to be taken for optimum outcome can certainly circumvent such an issue,” Kumar says.
He warns though that while the novel technology provides an alternate method for long-term protection, sustained drug delivery may not be that straightforward.
“Continuous presence and thus continuous exposure to drug may favour the possibility of development of drug-resistant organisms,” he emphasises.
This piece was produced by SciDev.Net’s Asia & Pacific desk.