By: Talent Ng’andwe
Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
[HONG KONG] A Chinese-produced vaccine to protect children against deadly Japanese encephalitis has been prequalified in safety and efficacy terms by the WHO. It is the first Chinese-made vaccine to gain WHO’s stamp of approval.
The vaccine, SA 14-14-2 manufactured by Chengdu Institute of Biological Products, is described by WHO as a “less expensive” option for protecting children at risk of contracting the disease, which infects around 70,000 people in Asia every year.
An estimated 10,000 to 15,000 deaths are reported in a population of more than 4 billion people who live in Japanese encephalitis-endemic regions in South-East Asia and the Western Pacific, according to the Program for Applied Technology in Health (PATH), a global health non-profit organisation that initiated clinical trials on the vaccine a decade ago.
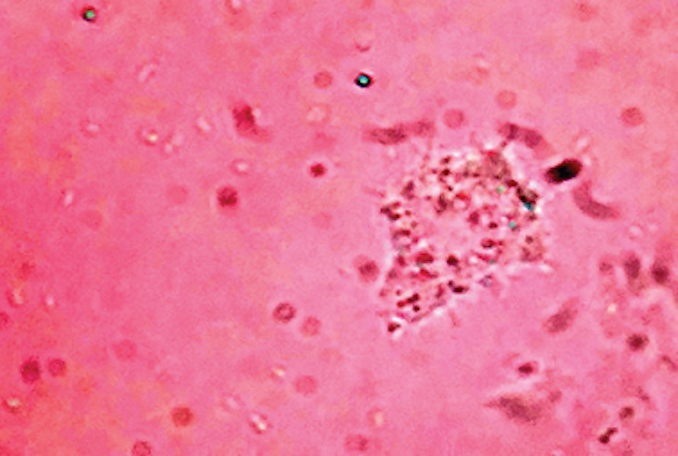
Japanese encephalitis is transmitted by a mosquito-borne virus. It infects the brain, leading to swelling that can kill or cause long-term disabilities, including paralysis, seizures and mental retardation.
The WHO prequalification paves the way for UN procurement agencies to purchase the vaccine, and serves as an endorsement of quality for countries in adopting it, according to PATH.
Kathleen Neuzil, director of PATH’s vaccine access and delivery programme, says it is the first time a Chinese vaccine manufacturer has achieved WHO prequalification, signaling China’s emergence onto the global vaccine marketplace.
“The growing capacity of China’s biotechnology sector and its emergence as an important vaccine supplier could help foster a more competitive vaccine manufacturing market,” Neuzil says.
The Japanese encephalitis vaccine is sold in a multi-dose presentation, but each child only receives one dose. The cost is similar in price to the measles vaccine, which costs less than US$0.30, she says.
Lingjiang Yang, Chengdu Institute's manager for international business, tells SciDev.Net the prequalification of the vaccine reflects the growing influence of pharmaceutical producers from emerging economies.
Tony Nelson, a pediatrician from the Chinese University of Hong Kong, says that while the Japanese encephalitis vaccine has been produced for some time in China for local use, it is significant that now the vaccine has undergone the regulatory requirements for WHO prequalification.
Nelson tells SciDev.Net that vaccines against Japanese encephalitis have existed since 1941, but they are expensive, produce undesirable side effects, and are not widely available.
This development “demonstrates that emerging market vaccine producers have the potential to produce vaccines at low cost, making them affordable for developing countries,” he says.
This article has been produced by SciDev.Net's South-East Asia & Pacific desk.