By: Anna Valmero
Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
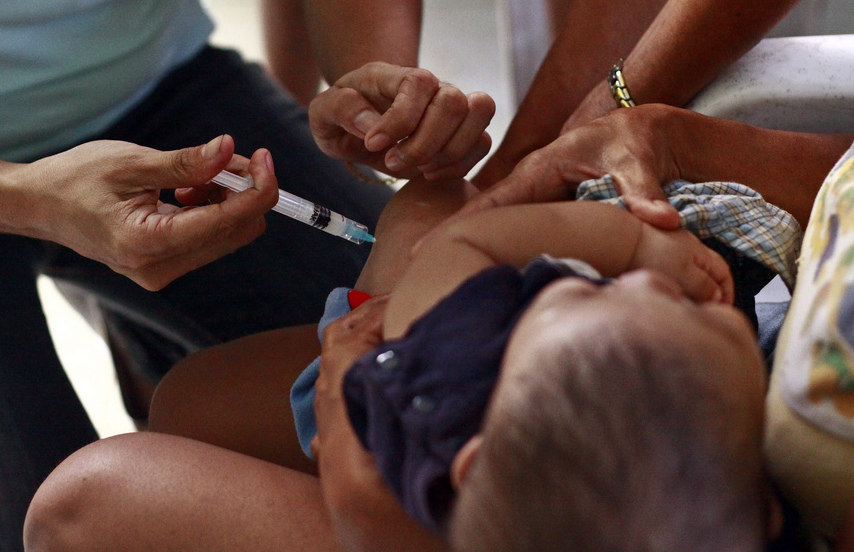
[MANILA] A controversial government scheme involving the purchase of pneumococcal conjugate vaccine (PCV) in the Philippines brings to focus on possible influences of drug firms on the country’s health programs and the need for transparency in decisions to gain public trust and support.
The Department of Health’s (DOH) controversial purchase in 2012 of the allegedly cheaper PCV 10 worth 833 million pesos (about US$18.54 million) prodded investigation of the contract as well as the resignation of health secretary Enrique Ona and the demotion of Eric Tayag from assistant secretary to director in late 2014.
Ona was quoted in Philippine reports that he had “good intentions” when he decided the purchase of PCV 10 that was sold a dollar cheaper in 2012 at US$15.40 than PCV 13 that cost US$16.34.
The main difference between PCV 10 and PCV 13 is the latter has immunisation coverage for three extra serotypes and can include patients above 50 years of age, in addition to children aged six weeks to five years old.
Antonio Dans, representative of the professional organisation Philippine College of Physicians (PCP), tells SciDev.Net that the decision on whether to buy PCV 10 or PCV 13 should be based on which is the more “effective” and “cost effective” option.
“(But) was it only scientific evidence that was involved in the decision of the controversial procurement of these vaccines or were other factors such as pharmaceutical influence at play?” asks Dans.
“The issue has put into light the existing problem of how pharmaceutical companies can actually affect health policies such as vaccine procurement and doctors’ choices in terms of drug prescriptions,” says Dans, a physician who also sits as president of the Philippine Society of General Internal Medicine, one of PCP’s member organisations.
“Doctors and policy makers in the health sector have to be familiar with the rules of evidence such as tapping cost-effective analysis to craft pro-patient policies,” Dans stresses.
PCV 10 versus PCV 13
Pneumonia is the second leading cause of infant mortality in the Philippines, with 2,628 deaths in 2010, according to official health statistics. The DOH added vaccination against pneumonia in its national expanded immunisation programme in 2013 in response to the WHO’s call.
“The expanded programme on immunisation has a national average of 80 per cent coverage. In remote rural areas such as the Autonomous Region in Muslim Mindanao, coverage is as low as 40 per cent,” explains Dans.
To make vaccination successful, 90 per cent or higher of the target population must be vaccinated to achieve herd immunity. Anything below this can lead to outbreaks, explains Dans.
The WHO recommends both PCV 10 and PCV 13 for national immunisation programmes against pneumonia. A cost-effective analysis (CEA) study can be tapped to identify which of the two can best address the immunisation needs of a country, argues a vaccine researcher who requested anonymity.
The vaccine researcher adds that a CEA study is an objective decision tool because it relies on different data parameters such as epidemiology, mortality rate, serotype distribution, direct and herd effects of vaccine, access to healthcare, vaccine cost, and injection equipment/cold chain cost. Each nation uses a different set of parameters to determine their vaccination programme.
An epidemiologist adds that existing researches that highlight the effectiveness of one PCV variant over the other are often authored by pharmaceuticals and results must thus be read very carefully.
Overall challenges
Dans suggests two ways to address pharmaceutical influences on public health programmes. First, there should be regulation on corporate favours to physicians such as junkets overseas that may affect drugs prescription.
Second, there should be better transparency measures in healthcare policies indicating potential conflict of interest when present and stating how they were addressed.
He also states the need to come up with clear implementing rules and regulations with regard to the Mexico City Principles endorsed by the international community in 2011 to regulate relationships of health practitioners with drug companies.
“For big procurements by the government, there is need for full disclosure so that the public is free to scrutinise the basis for the decision,” notes Dans.
In another interview, remedial lawyer Freidrick Vincent Lu of the Philippine Christian University notes that enacting the freedom of information (FOI) bill now pending in Congress will increase transparency and accountability in government processes.
“Without the FOI law, government projects are decided upon the discretion of officials and how they arrive at the decision is left out of public discussion. What can be done is to make public all the persons involved in biddings and acquisitions. This issue is not unique to the Philippines, it is observed in most developing nations,” says Dans.
Dans also urges the DOH to improve the salary of health professionals through the use of excise taxes collected from alcohol and tobacco products.
Each of the country’s 2,838 doctors handles about 18,000 patients, while the total nurse count of 4,576 means one nurse handles 11,000 patients, according to PCP research.
The country needs to address not only the supply of medicines or vaccines but strengthen the currently fragmented healthcare system. A functioning system needs to be both patient-driven and science based in action, Dans says.
Dans adds that the PCP is drafting a framework for the delivery of primary healthcare to promote preventive care instead of reactive care that is more costly. This type of system is what is practiced in the United Kingdom wherein patients through a robust health insurance system have access to private doctors.
As of this writing, results of the inquiry on the PCV 10 procurement are still pending with the National Bureau of Investigation. DOH spokesman Lyndon Lee Suy declined to comment on the issue pending results of the investigation.
This article has been produced by SciDev.Net's South-East Asia & Pacific desk.