By: Cristina Torres
Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
Dengue, a mosquito-borne disease, is a public health scourge that puts the health of billions of people at risk. It is estimated that it causes about 500,000 hospitalisations a year of mostly children. [1]
Control measures usually focus on programmes that require community involvement to improve sanitation and water management. The WHO has led the development of a global strategy plan to reduce dengue mortality and morbidity by 50 per cent by 2020. As there are still no comprehensive and effective measures for dengue prevention and control, current scientific initiatives focus on vaccine development and control of mosquito vectors.
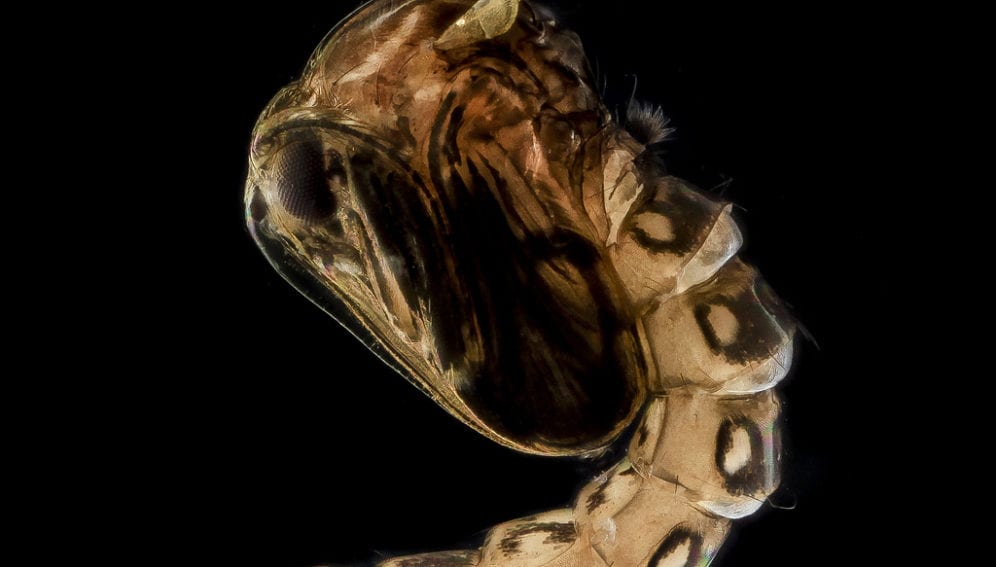
Vector control initiatives include suppressing the principal mosquito vector, Aedes aegypti. But more innovative vector control methods are currently being explored that make use of genetically modified (GM) mosquitoes developed under laboratory conditions and released in the environment.
One such research has been undertaken in Malaysia in collaboration with the UK-based firm Oxitec. It makes use of an engineered “genetically sterile” strain (OX513A) and a wild-type laboratory strain, comparing their performance in terms of longevity and dispersal when released in the environment to prepare for the future testing of “genetic control strategies”. [2]
Another research makes use of the Aedes albopictus Actin-4 gene, resulting in flightless female mosquitoes unable to bite. [3] Another bio-control approach in dengue prevention is currently being tested in Australia, Indonesia and Vietnam. This involves the release of A. aegypti infected with the wMel strain of Wolbachia, which are naturally occurring bacteria capable of reducing the ability of mosquitoes to pass dengue between people. [4]
“While there is unanimous agreement about the need for more effective dengue vector control, the moral dilemma is essentially about the uncertainty of the long-term impact of such interventions on humans and their possible ecological effects.”
Cristina Torres, University of the Philippines
While all these scientific research studies are initially developed in the laboratory, they have to be tested under real world conditions and released into the environment in dengue-endemic communities to prove their safety and effectiveness to address the dengue epidemic.
As these could potentially protect or harm humans and the environment, these research studies would have to be approved by local ethics committees in specific locations before they are conducted. In South-East Asia, members of research ethics committees that have reviewed the early phase protocols have agonised over the risks and benefits of such experiments.
While there is unanimous agreement about the need for more effective dengue vector control, the moral dilemma is essentially about the uncertainty of the long-term impact of such interventions on humans and their possible ecological effects. Generally, communities at risk are in favour of effective interventions for disease control, but they hesitate to be the guinea pigs for such studies. Indeed, the harm that could possibly result from field experiments are real enough to scare people.
Ethics reviews of community-based protocols have to comply with local laws and regulations as well as important international guidelines on biomedical research involving human subjects set by the World Medical Association and the Council for International Organizations of Medical Sciences. Both organisations define the responsibilities of research ethics committees related to the application of the principles of autonomy, beneficence and justice, the pillars of human subject protection framework. [5,6]
The determination of vulnerability of research participants and the benefit-risk assessment of research procedures are important assessment points about the probable effects of dengue researches on the human population. While the potential benefits are clear in relation to decreasing the burden of dengue incidence, the potential risks of releasing GM mosquitoes in communities may not be immediately discernible and its long-term effects may be difficult to predict.
The other important issue is to determine the appropriate procedures for informed consent for such studies, as the individuals who consented to the release of a GM mosquito cannot control the movement of the mosquito around their community and nearby areas. Is individual informed consent sufficient when the whole neighbourhood is going to serve as the experimental host environment for a GM organism? What type of community consultation should take place? Would permission by the community leader be sufficient to allow the initiation of the study?
Given these parameters for assessment, it will be difficult for research ethics committees to arrive at a unanimous decision because of the uncertainties involved about the future impact of GM mosquitoes. [7] The discussion could very well resemble the debates about GM food that could potentially address food shortage but whose long-term effects are not known.
The study on the Wolbachia experiment has seen the wisdom of involving social scientists in the team to perform the essential social preparation that community engagement requires. Before the community engagement could begin, it was necessary to get permission from national governments and to invite them as potential public health partners in the use of vectors for dengue prevention and control. It was also necessary to conduct consultations at various social levels, from the national government to the basic communities where the mosquitoes would be released.
In closing, the issue of biomedical research highlights the ethical and social dilemmas that arise when using community targeted interventions that should take into consideration local social structures, community beliefs and traditions. An ethically sound research should be culturally sensitive and respectful of people’s values and concerns.
Cristina Torres is a social science professor from the University of the Philippines Manila where she served as department chair and associate dean for academic affairs. She is currently the Asian regional coordinator of the Forum for Ethical Review Committees in Asia and the Western Pacific, a training and advocacy group set up by the WHO Special Programme for Diseases of Poverty.
This article has been produced by SciDev.Net's South-East Asia & Pacific desk.
References
[1] WHO Special Programme for Research and Training in Tropical Diseases (TDR) Scientific Working Group Report on Dengue (WHO, October 2006)
[2] Renaud Lacroix and others Open Field Release of Genetically Engineered Sterile Male Aedes aegypti in Malaysia (PLOS One, August 2012)
[3] Geneviève M. C. Labbé and others Female-specific flightless (fsRIDL) phenotype for control of Aedes albopictus (PLOS Neglected Tropical Diseases, July 2012)
[4] Francesca D. Frentiu and others Limited Dengue Virus Replication in Field-Collected Aedes aegypti Mosquitoes Infected with Wolbachia (PLOS Neglected Tropical Diseases, February 2014)
[5] World Medical Assembly (WMA) Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects (WMA, October 2013)
[6] Council for International Organizations of Medical Sciences (CIOMS) International Ethical Guidelines for Biomedical Research Involving Human Subject (CIOMS, 2002)
[7] Juntra Karbwang and Cristina Torres Ethical issues related to clinical trials outside the International Conference on Harmonization regions (Future Medicinal Chemistry, September 2011)